RonPaul2012
Hazard to Self
 
Posts: 89
Registered: 29-2-2012
Member Is Offline
Mood: No Mood
|
|
Experiments/reagents from produce.
I work on a pretty large farm and we are constantly composting tons of produce.
This year I plan to do some experiments with produce e.g make ethanol and other compounds ; just for fun and maybe to get some reagents.
I have access to large 55 gallon HDPE drums for fermentation and what not.
I am just posting this for fun and maybe to get some ideas.
Here is a list of the produce that I can get my hands on in large quantities: Tomatoes, Pumpkins, Spuash,White peaches,Yellow peaches, White
nectarines , yellow nectarines, Strawberries, Blackberries,Red raspberries,Black Raspberries, Every kind of apple under the sun,Zucchini, Cucumber,
White corn, Yellow corn, A lot of different kinds of peppers, And tomatillos.
There is probably some other stuff I forgot to mention 
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Apparently, silage efluent contains extremely high levels of nitrate, to the extent that you can crystallize nitrates from them and that NO2 gas can
accumulate in silos in dangerous levels.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Sweet! Corn:
| Quote: | Furfural is an organic compound derived from a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural
comes from the Latin word furfur, meaning bran, referring to its usual source.
Furfural is an heterocyclic aldehyde. Its chemical formula is OC4H3CHO.
In 1840, the Scottish chemist John Stenhouse found that the same chemical could be produced by distilling a wide variety of crop materials, including
corn, oats, bran, and sawdust, with aqueous sulfuric acid, and he determined the empirical formula (C5H4O2).
Except for occasional use in perfume, furfural remained a relatively obscure chemical until 1922, when the Quaker Oats Company began mass-producing it
from oat hulls. Today, furfural is still produced from agricultural byproducts like sugarcane bagasse and corn cobs.
For crop residue feedstocks, about 10% of the mass of the original plant matter can be recovered as furfural. Furfural and water evaporate together
from the reaction mixture, and separate upon condensation.
In the laboratory, synthesis of furfural from corn cobs takes place by reflux with dilute sulfuric acid. |
Organic Syntheses has an article on the method.

If you're feeling really ambitious!
Furural -> furan:

And then -> tetrahydrofuran (THF, the laboratory solvent):

|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Does anyone have any thoughts on what the reaction mechanism could be? ^
Might it have any similarities to sugar browning dehydration?
http://www.sciencemadness.org/talk/viewthread.php?tid=16766
|
|
|
RonPaul2012
Hazard to Self
 
Posts: 89
Registered: 29-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by peach  |
Sweet! Corn:
| Quote: | Furfural is an organic compound derived from a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural
comes from the Latin word furfur, meaning bran, referring to its usual source.
Furfural is an heterocyclic aldehyde. Its chemical formula is OC4H3CHO.
In 1840, the Scottish chemist John Stenhouse found that the same chemical could be produced by distilling a wide variety of crop materials, including
corn, oats, bran, and sawdust, with aqueous sulfuric acid, and he determined the empirical formula (C5H4O2).
Except for occasional use in perfume, furfural remained a relatively obscure chemical until 1922, when the Quaker Oats Company began mass-producing it
from oat hulls. Today, furfural is still produced from agricultural byproducts like sugarcane bagasse and corn cobs.
For crop residue feedstocks, about 10% of the mass of the original plant matter can be recovered as furfural. Furfural and water evaporate together
from the reaction mixture, and separate upon condensation.
In the laboratory, synthesis of furfural from corn cobs takes place by reflux with dilute sulfuric acid. |
Organic Syntheses has an article on the method.

If you're feeling really ambitious!
Furural -> furan:

And then -> tetrahydrofuran (THF, the laboratory solvent):
 |
This is what I'm talking about man 
I will keep this for reference once I can get my hands on some corn.
This sounds fun 
I do have a lot of dried indian corn from last year, do you think that will work ?
I forgot that I can also get a lot of sh...... I mean manure too.
[Edited on 22-3-2012 by RonPaul2012]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
If you use conc. HCl and have a second organic solvent phase present, you can get chloromethyl furfural directly from cellulose in a high yield.
Heating that with ethanol with reform HCl and result in the respective ethyl ether. Sounds pretty easy to me.
Why people waste so much effort on cellulosic ethanol I do not know... This way is far simpler and more efficient.
The 1.5 billion tons of biomass waste biomass made every year in the US would result in roughly 230 million tons of ethoxymethyl furfural after the
respective chloromethyl furfural is reacted with 13 billion gallons of ethanol (which happens to close to what we are producing now.) The total yield
would be around 50 billion gallons, and it has the same energy density as gasoline. Since it burns in diesel engines, it would displace almost
ALL of the US diesel consumption! Basically it would multiply our biofuel production 5 times over what it is now... with out even growing any
additional crops.
[Edited on 22-3-2012 by 497]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
For furfural;
| Quote: | Many plant materials contain the polysaccharide hemicellulose, a polymer of sugars containing five carbon atoms each. When heated with sulfuric acid,
hemicellulose undergoes hydrolysis to yield these sugars, principally xylose. Under the same conditions of heat and acid, xylose and other five carbon
sugars undergo dehydration, losing three water molecules to become furfural:
C5H10O5 → C5H4O2 + 3 H2O |
Quote: Originally posted by RonPaul2012  | This is what I'm talking about man 
I will keep this for reference once I can get my hands on some corn.
This sounds fun 
I do have a lot of dried indian corn from last year, do you think that will work ?
I forgot that I can also get a lot of sh...... I mean manure too.
[Edited on 22-3-2012 by RonPaul2012] |
Glad you liked it.
It'll work from numerous fibre crops, so you'll likely be fine with whichever corn.
I wouldn't be surprised if this was usually done with just the cob, after the corn it's self had been stripped and sold. I would expect the cob it's
self is richer in the cellulose than the grain, as it's very fibrous by comparison. It also has less water in it, which will make it easier to
maintain a decent acid concentration versus mass of substrate going in.
If you could find someway to rip the cobs to shreds, that'd be good.
With the manure, you could leach that, mix it with straw, pasteurise, grow mushrooms on it and then harvest things from the mushrooms. Or, leach the
entire lot.
Something I'd like to try is repeating the discovery of phosphorus. Which was found by a German alchemist called Brand. He was trying
to make the philosophers stone. His theory was that, since pee can be golden coloured and comes from humans, it might be in that. So he evaporated off
about 1000l of pee (diligently collected and left to rot in the meantime) to obtain the dissolved content, which he then distilled at high
temperatures. The yield was something that stank, glowed in the dark and easily caught alight; phosphorus.
It may also work with the leach from manure; which will be full of phosphates.
| Quote: | | Furfural was first isolated in 1821 (published in 1832) by the German chemist Johann Wolfgang Döbereiner, who produced a small sample as a byproduct
of formic acid synthesis.[3] At the time, formic acid was formed by the distillation of dead ants, and Döbereiner's ant bodies probably contained
some plant matter. |
Do you have any ants on the farm? 
|
|
|
RonPaul2012
Hazard to Self
 
Posts: 89
Registered: 29-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by peach  |
For furfural;
| Quote: | Many plant materials contain the polysaccharide hemicellulose, a polymer of sugars containing five carbon atoms each. When heated with sulfuric acid,
hemicellulose undergoes hydrolysis to yield these sugars, principally xylose. Under the same conditions of heat and acid, xylose and other five carbon
sugars undergo dehydration, losing three water molecules to become furfural:
C5H10O5 → C5H4O2 + 3 H2O |
Quote: Originally posted by RonPaul2012  | This is what I'm talking about man 
I will keep this for reference once I can get my hands on some corn.
This sounds fun 
I do have a lot of dried indian corn from last year, do you think that will work ?
I forgot that I can also get a lot of sh...... I mean manure too.
[Edited on 22-3-2012 by RonPaul2012] |
Glad you liked it.
It'll work from numerous fibre crops, so you'll likely be fine with whichever corn.
I wouldn't be surprised if this was usually done with just the cob, after the corn it's self had been stripped and sold. I would expect the cob it's
self is richer in the cellulose than the grain, as it's very fibrous by comparison. It also has less water in it, which will make it easier to
maintain a decent acid concentration versus mass of substrate going in.
If you could find someway to rip the cobs to shreds, that'd be good.
With the manure, you could leach that, mix it with straw, pasteurise, grow mushrooms on it and then harvest things from the mushrooms. Or, leach the
entire lot.
Something I'd like to try is repeating the discovery of phosphorus. Which was found by a German alchemist called Brand. He was trying
to make the philosophers stone. His theory was that, since pee can be golden coloured and comes from humans, it might be in that. So he evaporated off
about 1000l of pee (diligently collected and left to rot in the meantime) to obtain the dissolved content, which he then distilled at high
temperatures. The yield was something that stank, glowed in the dark and easily caught alight; phosphorus.
It may also work with the leach from manure; which will be full of phosphates.
| Quote: | | Furfural was first isolated in 1821 (published in 1832) by the German chemist Johann Wolfgang Döbereiner, who produced a small sample as a byproduct
of formic acid synthesis.[3] At the time, formic acid was formed by the distillation of dead ants, and Döbereiner's ant bodies probably contained
some plant matter. |
Do you have any ants on the farm?  |
I
have seen a few ants here and there but it would take me a very long time to collect a few thousand. 
I'm so psyched about all of this 
Please post anymore experiments you can think of.
I was thinking of maybe getting acetic acid from apples.
If all goes well and I get some time on my hands I will document the synthesis of furfural and anything else you guys can think of 
Thanks peach
[Edited on 23-3-2012 by RonPaul2012]
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Depends somewhat on the source of the manure. Chicken manure may have reasonable phosphate content, like 2% P2O5 assay by dry weight (and is generally
pretty rich in fertilizer chemicals compared to other manures). Cow manure on the other hand... about a tenth of that.
http://www.nhm.ac.uk/research-curation/research/projects/pho... for more than you probably need to know. This is why my nitre bed project used all
chicken and duck manure. As I recall humans are unusual among animals in that they excrete phosphorus compounds in their urine...
The less you bet, the more you lose when you win.
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Extract some of the spice from hot peppers.
You can use it as tear gas. Drop a mL on a stove, turn the stove on, leave. Works every time!
ʃ Ψ*Ψ
Keepin' it real.
Check out my new collaborated site: MNMLimpact.com
|
|
|
RonPaul2012
Hazard to Self
 
Posts: 89
Registered: 29-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by GreenD  | Extract some of the spice from hot peppers.
You can use it as tear gas. Drop a mL on a stove, turn the stove on, leave. Works every time! |
I have always
wanted to isolate Capsaicin.
Here is a link from this forum on it http://www.sciencemadness.org/talk/viewthread.php?tid=5329
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Intriguing. I found a note about this and farm animals:
| Quote: | | Phosphatic Calculi - The hard masses are normally formed because of an environment of high-concentrate feed, low-roughage, feed with low
calcium-to-phosphorus ratio, high magnesium diets and alkaline urine. A diet mainly made up of grains results in urine with large amounts of
phosphorus in it. |
That's interesting because, more recently, the combine harvester, high speed grain mill, herbicides and insecticides have allowed humans to begin
consuming orders of magnitude more grain based product than they would have been not all too long along; it's used as filler in many processed foods.
A farm can now harvest a weeks worth of work for a small group of men in an hour or two with just one or two guys and the harvester.
And this note about possibly mining human waste;
| Quote: | Associate Professor Cynthia Mitchell, of the Institute for Sustainable Futures at the University of Technology, Sydney (UTS), said the world's
deposits of phosphorus are due to run out in about 50 years.
"Urine is the most concentrated source of phosphorus," she said. "At the moment we dilute that through our sewage system and send it out to the ocean.
|
Anomous has a nice video of peppers being extracted:
<iframe sandbox width="640" height="480" src="http://www.youtube.com/embed/CYyDVZPaZng" frameborder="0" allowfullscreen></iframe>
Peppers can be kind of expensive. If one takes themselves to the Indian cooking essentials sections of the supermarket, there are usually bags of
dried chilli.
I'll happily jalapeño's straight out the jar for breakfast, but this bag has lasted at least a year. 300g, bone dry, is a lot! Only costs a few
pounds a bag. I would predict there's about 1 to 10g of capsaicinoids in there.

That's about enough that people will start complaining about things being too hot.

[Edited on 23-3-2012 by peach]
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
I assume, failing possesion of a soxhlet, that the capsaicinoids could be extracted via reflux of the solid over solvent?
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by RonPaul2012  | I have seen a few ants here and there but it would take me a very long time to collect a few thousand. 
[Edited on 23-3-2012 by RonPaul2012] |
Bury a glass bottle with sugar in the bottom of it next to an ant hill and it should fill up in short order as long as they can't grip the side of the
bottle.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hexavalent  | | I assume, failing possesion of a soxhlet, that the capsaicinoids could be extracted via reflux of the solid over solvent? |
Yep, but you'll end up using more solvent than the soxhlet would.
You could also stick an equalizing addition funnel on top of the flask, then a condenser on top of that. Lightly pack the funnel with plant mass. Let
the funnel flood with solvent, open the tap to drain it, close it, flood, drain... or let it flood and then try to balance the drain rate with the
condensation rate.
Quote: Originally posted by Sedit  |
Bury a glass bottle with sugar in the bottom of it next to an ant hill and it should fill up in short order as long as they can't grip the side of the
bottle. |
Entrantment? You filthy beast. 
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Apple --> Alcohol --> Fuel
Purify and sell as a fuel or fuel additive for gasoline.
Normally, the economics don't work (for example, given the cost of energy needed to produce the nitrates, etc.) but as these are apples that otherwise
would not be sold, it may be worth your time.
There may even be government subsidiaries for the production equipment, etc.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Capsaicin extraction from Red Chili pepper seeds only
I wasn't sure if I should post this here of in the Capsaicin extraction thread but seeing as how the topic has popped up here here goes!
I have been doing lots of extractions and was interested in the extraction of Capsaicin, when researching though I found that many preps suggested to
use the pepper flesh as well as the seeds. I was going to do this however upon furthur reading I realized that many preps and references mention the
extraction of many unwanted products which are also soluble in the extraction solvent (in my case Ethanol) such as pigments etc.
Just for fun i decided to strip the seeds out of some red chili peppers and dry them, and extract only the seeds and accept lower yields as a result
and see what I ended up with.
This was fun and I am still not done evaporating the concentrate, I have made a quick word document with photos (very rough copy at the moment) to
post 
The word document was too large (7mb) to attach on the forum, I had to find a file hosting service. This service sends a download link to your email
and then you simply click the link and download the Microsoft word file. If anybody knows how I could attach such a document to the forum please let
me know!
Anyways, the document can be found here:
http://www.filehosting.org/file/details/324247/Capsaicin_Ext...
|
|
|
RonPaul2012
Hazard to Self
 
Posts: 89
Registered: 29-2-2012
Member Is Offline
Mood: No Mood
|
|
Speaking of mushrooms , I found this massive tree fungus while I was weed eating.
Any ideas ?
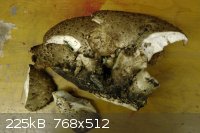
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Tree fungus, eh? Presumably a polypore. The underside of the mushroom is often more helpful than the top in identification (though in this case I
dunno if it would help). Take a look at http://americanmushrooms.com/polypores.htm and see if any of them looks like a close match.
The less you bet, the more you lose when you win.
|
|
|
RonPaul2012
Hazard to Self
 
Posts: 89
Registered: 29-2-2012
Member Is Offline
Mood: No Mood
|
|
From what I can tell from the top and bottom it looks a lot like this one http://americanmushrooms.com/taxa/Polyporus_craterellus_01a.... .
After checking a few pictures on the internet I found that this one http://americanmushrooms.com/taxa/Polyporus_squamosus_03.htm looks like the one I have and it matches most discriptions too , but the pores are
slighlty off.
Does anybody know if I can do anything with this ?
If not , I was either going to throw it away or make an Omelette with it , provided that I won't drop dead in doing so 
[Edited on 12-4-2012 by RonPaul2012]

[Edited on 12-4-2012 by RonPaul2012]
|
|
|
RonPaul2012
Hazard to Self
 
Posts: 89
Registered: 29-2-2012
Member Is Offline
Mood: No Mood
|
|
Since you guys didn't respond I ended up eating this demon of deliciousness .
I still have about half of it.
It tastes like some type of exotic seafood 
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
I accidentally stumbled on an interesting paper describing the various products that can be easily produced by (catalyzed) biomass pyrolysis. Since
most people have easy access to biomass and fire, I thought there was an above average chance of someone being able to use this info. I'm sure there
is more scattered around the forum already.
https://docs.google.com/viewer?a=v&q=cache:b0eAXXY15bQJ:...
From cellulose:
Furfural, up to 80% of organic condensate using ZnCl2 or MgCl2 catalysts.
Activated carbon, as a co-product of furfural production
Acetic acid, as the main volatile co-product when using ZnCl2 catalyst.
Levoglucosan, Levoglucosenone, 1-hydroxy-3,6-dioxabicyclo[3.2.1]octan-2-one, up to 30% yields using various acid catalysts
From lignin:
Aromatic hydrocarbons, up to 90% of organic condensate (32% toluene, 33% xylene) using zeolite catalyst bed after the pyrolysis chamber.
Syringol, up to 10% yield vs dry wood, no catalyst
Guaiacol, up to 3% yield vs dry wood, no catalyst
Various cresols, and other substituted phenols in smaller amounts, but even 4-propenylsyringol has been produced in usable amounts.
Cracking the pyrolysis gas with Pd catalyst is said to increase phenolic yields.
https://docs.google.com/viewer?a=v&q=cache:gqvup7b4uiAJ:...
They get good yields of syringol, guaicol here.
http://www.sciencedirect.com/science/article/pii/S1383586600...
More good syringol yields, and fractional distillation brings it to 92% pure.
http://www.sciencedirect.com/science/article/pii/S0961953497...
http://www.sciencedirect.com/science/article/pii/S0960852411...
These talk about purification of the products.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Furfural is useful for forming resins, either by itself or with additives. It polymerizes under mildly acidic conditions to a dark colored polymer.
Interestingly, this new polymer still retains all it carbon-carbon double bonds. It is actually a condensation reaction, and water is released.
The furan rings become bridged by methylene groups attached on the carbon atoms of the ring adjacent to the oxygen in the furan ring.
Since furfural is an aldehyde, it can also condense with amines to form resins, in a different type of reaction. US Patent 7781521 describes
furfural-urea resins. These resins can contain a range of different proportions, because the furfural does not need any urea to polymerise.
Apparently plain fural does not undergo polymerisations because of its aromatic character, unlike turpenoid resins which contain carbon-carbon double
bonds that do polymerise. These natural plant turpenoids typically include Pinenes and Limonene, for example, which harden to become amber.
The cross-bonding in amber is not unlike that in polyethylene plastic.
|
|
|
RonPaul2012
Hazard to Self
 
Posts: 89
Registered: 29-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 497  | I accidentally stumbled on an interesting paper describing the various products that can be easily produced by (catalyzed) biomass pyrolysis. Since
most people have easy access to biomass and fire, I thought there was an above average chance of someone being able to use this info. I'm sure there
is more scattered around the forum already.
https://docs.google.com/viewer?a=v&q=cache:b0eAXXY15bQJ:...
From cellulose:
Furfural, up to 80% of organic condensate using ZnCl2 or MgCl2 catalysts.
Activated carbon, as a co-product of furfural production
Acetic acid, as the main volatile co-product when using ZnCl2 catalyst.
Levoglucosan, Levoglucosenone, 1-hydroxy-3,6-dioxabicyclo[3.2.1]octan-2-one, up to 30% yields using various acid catalysts
From lignin:
Aromatic hydrocarbons, up to 90% of organic condensate (32% toluene, 33% xylene) using zeolite catalyst bed after the pyrolysis chamber.
Syringol, up to 10% yield vs dry wood, no catalyst
Guaiacol, up to 3% yield vs dry wood, no catalyst
Various cresols, and other substituted phenols in smaller amounts, but even 4-propenylsyringol has been produced in usable amounts.
Cracking the pyrolysis gas with Pd catalyst is said to increase phenolic yields.
https://docs.google.com/viewer?a=v&q=cache:gqvup7b4uiAJ:...
They get good yields of syringol, guaicol here.
http://www.sciencedirect.com/science/article/pii/S1383586600...
More good syringol yields, and fractional distillation brings it to 92% pure.
http://www.sciencedirect.com/science/article/pii/S0961953497...
http://www.sciencedirect.com/science/article/pii/S0960852411...
These talk about purification of the products. |
Wow that's great , maybe one day I can try some of these
experiments out 
Thanks 497 
|
|
|