caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
DPT: an amateur vs pros
One wise boy found a way to make DPT with good yield. Here is the link: http://imploders.ru/dinitro-pentametilentetramin/2/ .Brief interpretation for these, who do not know russian. 50 ml HNO3 70% + 20 ml H2SO4 92,4%
cooled down to 0 C. Into this mixture 25 gr sulphamine acid NH3SO3 was added witn continious stirring. After 20 minutes hexamine dinitrate 27 gr was
added in small portions with stirring, temperature was kept below 15 C. After 40 minutes at 5-10 C mixture was slowly heated up to 30 C. Heating was
stopped, but temperature was growing up. Wheh it had reached 40 C, mixture was cooled down to 35 C. This op has been made few times. When an exotermic
reaction had finished (one our after initial heating), reaction mixture was diluted 1:2 by half-frosen solution of NH4NO3 (10%). Then acid was
neutralised with NaHCO3 to pH 6. During this op DPT precipitated. It was washed by water and isopropil alcohole. Author claims that yield is above 90
%.
Women are more perilous sometimes, than any hi explosive.
|
|
|
woelen
|
Thread Pruned
4-3-2012 at 23:29 |
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
DPT (3,7-dinitro-1,3,5,7 tetraazabicyclo [3,3,1] nonane)
The best method of obtaining DPT consists in introducing hexamine dinitrate to 90% sulfuric acid at 8-15 °C. After 45 minutes all is poured on ice
and the solution is filtrated. The filtrate is neutralized with 28% conc. ammonia to pH = 5.5-6.5 and DPT precipitated.
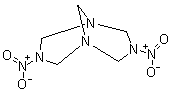
Since DPT is itself an explosive and can be prepared from a mixed H2SO4/HNO3 nitration on hexamine, it is surprising that this compound is not more
popular.
Here is a translation of Experiment number 1 from that russian page in the link:
| Quote: |
Dinitro-pentamethylenetetramine
To 100 ml of HNO3 (density 353g/ml or 53% conc.), cooled to +10 °C, was sprinkled 50g of sulfamic acid, a temperature rise is not observed. After
mixing with stirring at 10° C for 30 minutes, 53g of DNU was made, the temperature is kept below 20 °. After holding at this temperature for 20
minutes, the reaction mixture was warmed to 30 °, then external heating was discontinued, but the reaction continued to increase in temperature by
itself. After reaching 35-36 ° it was carried off to be gently cooled to 30 °C under running tap water. The temperature again continued to increase
after reaching 35-37 °, and the mixture was again cooled to 30-33 °, and so on for as long as the temperature kept increasing. The reaction mixture
was cooled to 15 °, and it becomes more viscous during this time. After dilution with 300ml of cold water, neutralization of sodium bicarbonate was
carried out to pH = 6, by the end of the neutralization of the solution became dull, and formed a number of fine-grained sediment. After an exposure
of 1 hour the solution was filtered, the precipitate was washed first with water and finally with isopropyl alcohol to facilitate drying. The mass of
dry product was 3.5g or about 8% of the theoretical value (assuming the formation of DPT 1 mol to 1 mol DNU). The nature of combustion of the sample
did not differ from the standard DPT. The measured melting point temperature of the sample amounted to about 195-198 °C. DPT obtained by the other
more traditional method has a melting point of 200-201 °C.
|
Please let me know if there are errors in my translation.
I do not know russian very good 
Obviously "Dinitro-pentamethylenetetramine" is just another name for DPT. The site discusses making DPT from sulfamic acid, H2N-SO3H.
[Edited on 18-3-2012 by AndersHoveland]
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
You should read all four pages! (change last digit in link or simple go to another pages of that thread) The best method had number 9. That boy
discussed 4 methods of DPT syntesis. His own method gives much better yield than another ones. Your translation is good, but you shouldn't spend time
and efforts for translation of the first and not the best experiment. I've translated description of experiment number 9- the best one, that gave
yield above 90%. There are some remarcable moments- concentration of HNO3 is crucial. 70% HNO3 gives the best yield, 63% (aseotropic, the usual
concentration) is lots worse. 99% is not the best variant too. The most remarkable here is the fact that this method was discovere by an amateur, a
kitchen chemist like many people at this forum.
Women are more perilous sometimes, than any hi explosive.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Experiment 8
The concentration of nitric acid was about 63% (density 1.386g/ml). A mixture of 50 ml of nitric acid and 35 ml of sulfuric acid (92.5% concentration,
density 1.825g/ml), which was cooled to 0 °C, was introduced with stirring 25 g of sulfamic acid. The temperature rose to 5 °C. After maintaining
the temperature for 20 minutes at 0 °C, 27g of DNU started to be added in small portions with stirring, keeping the temperature below 15 °C. The
reaction mixture was then kept at 5-10 °C for 40 minutes, while continuing stirring. The mixture was then gently heated to 30 °C, then heating was
stopped, but the temperature continued to increase by itself, as in the previous experiments. Just as before, the temperature was allowed several
times to reach 38-42 °C, and at such times the temperature was brought back down to 35 °C by cooling. When heat ceased to occur (in about an hour
after heating to 30 °C), the reaction mixture was cooled to 15 °C, while it thickened and became similar to the reaction mixture during cooking.
More PETN was diluted 1 to 2 cold porridge, prepared in advance by the freezing of 10% aqueous solution of ammonium nitrate. This was followed by
neutralization with sodium bicarbonate to a pH of ~ 6, resulting in much sediment. This was filtered, then washed with water and isopropanol. After
drying, the mass of sludge was 14.2g, or 65.1% of theory (DPT 1mol to 1mol DNU). The melting temperature of the product was 194-198 °C.
Experiment 9
The concentration of nitric acid 70% (density 1.411g/ml). All procedures are identical to experiment number 8, but with the following differences: the
amount of sulfuric acid is reduced to 20 ml, after the termination of heat the reaction mixture of the porridge thickens much faster and at lower
temperature, no more than about 25-30 ° C. The yield of DPT in this experiment was 21 g or 96.3% of the theoretical (assuming the formation of 1mol
DPT to 1mol DNU). When doing this experiment two additional times, the yield was 20g and 20.5g, respectively, which was consistently above 90% yield.
The products obtained by this method were in the temperature range of 198 ° C.
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  | When heat ceased to occur (in about an hour after heating to 30 °C), the reaction mixture was cooled to 15 °C, while it thickened and became
similar to the reaction mixture during cooking. More PETN was diluted 1 to 2 cold porridge, prepared in advance by the freezing of 10% aqueous
solution of ammonium nitrate.
|
With all my respect, small correction of your translation. Aforementioned boy meant, that reaction mixture became like a porridge, that occurs diring
PETN cooking. Then this porridge was diluted by half- frosen (mixture of liquid and ice crystalls) 10% solution of NH4NO3. I think, that method, that
you described (hexamine dinitrate and sulphuric acide) is good too- it does not require nitric acide at all. Low yield is not a great problem, may be
large amout of some by-products.
Women are more perilous sometimes, than any hi explosive.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Perhaps a perchlorate salt of DPT is possible?
C5H10N6O4*HClO4
Still not great oxygen balance, but it could be easy to make (just react DPT with dilute perchloric acid).
Also to mention, DPT can also be used as a precursor to HMX. If one wants to minimise the ammount of 99% HNO3 or acetic anhydride that needs to be
used, the DPT can be made first from normal mixed acids, then nitrated further.
see the other thread in this forum, "HMX by way of nitration of dpt"
https://sciencemadness.org/talk/viewthread.php?tid=738
[Edited on 25-3-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
from E+W forums archive:
| Quote: |
Ive heard of Dinitropentamethylenetetramine. Is it an explosive? You can make it with HDN and H2SO4. You can convert this to Hexogen but im interestet
in DPT itself. Has anyone experiences with it?
HDN [hexamine dinitrate] preparing from book "Lab preparing of nitrocompounds" by Orlova E.Y.:
Dissolve 1 part hexamine in 1.5 parts of water. Then add 50-60% nitric acid (at 15-20C) in quantity sufficient to receive a 20% waste NA.
Filter crystalls and wash it with absolute ethanol. Dry it at 60C.
About DPT:
I think the best way to prepare it is described in US pat 4,338,442. It also possible to make from hexamine and nitrourea with better yeild but I have
no details.
DPT has poor stability to hydrolysis. DPT is a intermediate in HMX synthesis.
It possible to make a HMX with 75% yeild from DPT, NA [nitric acid] and ammonium nitrate. |
Apparently DPT can be detonated, but has a low sensitivity:
| Quote: |
Among low sensitivity substances are non—cap explosives: dinitro benzene, dinitropentamethylenetetramine, ANFO, ammonium nitrate,
nitromethane, ...
Safety of Reactive Chemicals and Pyrotechnics By Tadao Yoshida, Yūji Wada, Natalie Foster
|
DPT forms monoclinic [rectangular prism, with parallelogram as its base] crystals.
"The crystal structure of dinitropentamethylenetetramine (DPT)",C. S. Choi and S. Bulusu, Acta Cryst. (1974). B30, 1576-1580
[Edited on 25-3-2012 by AndersHoveland]
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AndersHoveland  | Perhaps a perchlorate salt of DPT is possible?
C5H10N6O4*HClO4
Still not great oxygen balance, but it could be easy to make (just react DPT with dilute perchloric acid).
Also to mention, DPT can also be used as a precursor to HMX. If one wants to minimise the ammount of 99% HNO3 or acetic anhydride that needs to be
used, the DPT can be made first from normal mixed acids, then nitrated further.
see the other thread in this forum, "HMX by way of nitration of dpt"
https://sciencemadness.org/talk/viewthread.php?tid=738
[Edited on 25-3-2012 by AndersHoveland] |
Is DPT a base? May be situation with this compound like one with urea- urea itself is a base and some salts of it are well-known (nitrate), but
nitrourea is a weak acide, to say nothing about dinitrourea (thanks, Anders, for pdf about dinitrourea). DPT is a precursor to HMX, and moreover
according one russian article nitration of DPT can be performed with HNO3 plus AN without Ac2O. What is most remarkable, this process doesn't require
large amount of HNO3- 3.2 mole HNO3 + 1.6 mole of AN per one mole of DPT. Treatment is to be performed at 60-65 C.
Women are more perilous sometimes, than any hi explosive.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by caterpillar  |
Is DPT a base? May be situation with this compound like one with urea- urea itself is a base and some salts of it are well-known (nitrate), but
nitrourea is a weak base |
DPT contains three tertiary amines (trimethylamine is another example of a tertiary amine). In the molecule of DPT, the nitramine groups will have no
effect on the ability of the other amines to act as a base. In other molecules, such as nitroguanidine or 2-nitroaniline, the nitro groups do make the
amines less basic because the amines are electron donating to the nitro groups.
This does not mean that all three amines will be able to act as a base. The amines are all in close proximity to eachother, so probably only one of
them will be able to hold a positive charge when it is protonated, possibly two, but I think this would be more doubtful. Hexamine, for example, can
only act as a base towards two hydrogen ions. (you might have read about many metal atoms holding double or triple positive charges, but other factors
are involved, usually complexation with water molecules or crystal lattice energies)
[Edited on 27-3-2012 by AndersHoveland]
|
|
|
|