| Pages:
1
2
3 |
DrEntheogen
Harmless

Posts: 18
Registered: 1-2-2012
Member Is Offline
Mood: Reacted
|
|
Ideas for new hallucinogens?
Everyone is always following "Recipes" to make something they want or they just want to try the reactions.
Instead of following everyone else why cant we take something thats "legal" rearange the molecular structure to make something thats still "legal" (or
instead of legal we can say uncontrolled ) but psychoactive. ) but psychoactive.
My example is Leonorine the structure is pictured below.
Their has to be some potential for this. What does everyone think? What would/could you do to make this more psychoactive and become a full agonist of
a receptor that would cause hallucinations and a feeling of well being and happines all over? Is there any other molecules, compounds, alkaloids, etc.
that anyone has thought this about or would look at diffarentley?

"Orbitals are for mathematicians - Organic chemistry is for people who like to cook!" - Alexander Shulgin.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
First of all, that is not the structure of leonorine. That compound is called leonurine and I would like to know why are you spreading this
misinformation on the internet? What is the reference for your claims that this compound is a hallucinogen?
There are at least 24 scientific articles published in regard to the physiological or chemical aspects of this compound and not a single one mentions
anything that would lead to a conclusion about any hallucinogenic properties. There are a few Chinese herbal drugs that contain it and these have been
used for centuries, yet no reports about any hallucinogenic side effect!
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
I know from personal and tertiary experience that it can induce vivid dreams at higher doses
[Edited on 2-15-2012 by AirCowPeaCock]
BOLD
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Quote: Originally posted by AirCowPeaCock  | I know from personal and tertiary experience that it can induce vivid dreams at higher doses
[Edited on 2-15-2012 by AirCowPeaCock] |
probably metabolites.
OP: Aren't you the kid asking us for information on what chemistry is and what molecules are, and here you are, trying to think of novel drugs?
Do us all a favor and stop posting this kind of info. You can look up Chris Nichols, as he is your man. Find his papers and read all of them, then
return.
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
read pihkal and tihkal, there is a lot to be learned about developing psychoactive compounds.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Do you mind describing its synthesis or at least provide the characterization data? You sure don't expect anyone is going to believe such
unsubstantiated claims.
|
|
|
DrEntheogen
Harmless

Posts: 18
Registered: 1-2-2012
Member Is Offline
Mood: Reacted
|
|
Quote: Originally posted by Nicodem  | First of all, that is not the structure of leonorine. That compound is called leonurine and I would like to know why are you spreading this
misinformation on the internet? What is the reference for your claims that this compound is a hallucinogen?
There are at least 24 scientific articles published in regard to the physiological or chemical aspects of this compound and not a single one mentions
anything that would lead to a conclusion about any hallucinogenic properties. There are a few Chinese herbal drugs that contain it and these have been
used for centuries, yet no reports about any hallucinogenic side effect! |
You have miss understood what ive said. i said that its psychoactive, and if you re read my post im curious about what anyone would do to
change/rearange the molecular structure to make it produce hallucinations. theirs no where that ive said it causes hallucinations. the reason for this
thread is example THC is a mild verry mild at that hallucinogen, its psychoactive and it should be possible to rearange/change its structure so it
could produce hallucinations, more euphoria etc. re read the post. theres no need to come at my neck, im only trying to start a discussion about
things that are legal and can be made into a hallucinogen, empathogen/entactogen and stay legal. and your right about the spelling LEONURINE, but
thats about all. i misspelled the name. and i also wanted anyone to post about this or anyother legal alkaloid, compound etc. that they think could be
used to do the same thing.
"Orbitals are for mathematicians - Organic chemistry is for people who like to cook!" - Alexander Shulgin.
|
|
|
DrEntheogen
Harmless

Posts: 18
Registered: 1-2-2012
Member Is Offline
Mood: Reacted
|
|
and for the record i would never post nor tell anyone something that i wasnt 100% sure about and that i didnt have personal experience or a reference
for.
"Orbitals are for mathematicians - Organic chemistry is for people who like to cook!" - Alexander Shulgin.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
If you have a veritable reference, then post it already like you were told to do.
| Quote: | | the reason for this thread is example THC is a mild verry mild at that hallucinogen |
THC as a very mild hallucinogen? You must be kidding. That one is among the most potent hallucinogens known to man, both by dosage and effect. Well,
perhaps you just did not took enough?
| Quote: | | theres no need to come at my neck, im only trying to start a discussion about things that are legal and can be made into a hallucinogen,
empathogen/entactogen and stay legal. |
You want to start a discussion, yet I see no reference to any article worth discussing here. Instead you give us insignificant speculations about how
legality is possibly connected with hallucinogenic properties of compounds. That is ridiculous. This is a chemistry forum and some of us take science
seriously. So if you want to discuss the structure-activity relationship and receptor affinity of various types of hallucinogens, that is fine and
your are welcome to do so as long as you cite sources, but if you want to discuss about unreferenced speculations, then you joined the wrong forum.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
On the chemistry side of things, Dr. Nichols from Purdue has published a lot of interesting work. He designs ligands for the 5HT2A receptor by mapping
out the binding site. In fact, many of his creations are becoming quite popular hallucinogens (25i-NBoMe series). They are very, very potent agonists
for the 5HT2A receptors with binding affnities (Ki) in the nM range.
This here is an excellent lecture by Dr. Nichols: Advances in Understanding how Psychedelics Work in the Brain
There seem to be two pathways initiated by the serotonin 2A receptor. Nichols goes into detail, but basically there is the IPI3 pathway which is your
standard inisitol cascade causing Ca2+ release from the ER and you've got this other strange arachidonic acid pathway.
It's fascinating how important this is to the effects of psychedelics, because at a molecular level the differences are made. One molecule may have a
shape that twists and contorts the receptor in a way that activates the IPI3 pathway more than another. This is for one receptor. Years of work have
been compiled on this single receptor (5HT2A). Other hallucinogenic receptors include the Kappa-opioid (Salvia divinorum and its terpenoids are
agonists for this receptor), the NMDA receptor (dissociatives are antagonists), the sigma/trace-amine receptors (DMT, antidepressants and some
dissociatives function on this receptor type) and many more. Alexander Shulgin and Nichols work by making minute modifications to the structure of
molecules to determine the induced effect of said changes. As this is a (primarily) chemistry forum, I suppose you may be interested in that kind of
work. The neurobiology is more complex and more murky because neurobiology must take into account all of these receptors and translate their
activities into psychedelic and physiological effects.
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
| Quote: | | THC as a very mild hallucinogen? You must be kidding. That one is among the most potent hallucinogens known to man, both by dosage and effect.
|
I can't agree Nicodem ─ by itself, THC is quite mild by comparison to, say, LSD or psilocybin . . .
Some (fast-disappearing) low-latitude sativas are certainly hallucinogenic, but this is due to other relatively less investigated compounds such as
THCV which potentiate THC's ability to produce hallucination!
I used to cultivate, years ago, sativas which would produce a 'trip' that would often be a helter skelter experience (sigh)!
P
"I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones"
A Einstein
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pulverulescent  | | Quote: | | THC as a very mild hallucinogen? You must be kidding. That one is among the most potent hallucinogens known to man, both by dosage and effect.
|
I can't agree Nicodem ─ by itself, THC is quite mild by comparison to, say, LSD or psilocybin . . . |
That is a bit hard to compare, because the main effect of LSD and psilocibin is a psychedelic experience while THC does not have any psychedelic
effect (at least not to the majority of people). Besides, the hallucinations caused by LSD and psilocibin are mainly visual while THC causes primarily
spatio-temporal hallucinations and closed eye visuals. Furthermore, THC impairs cognitive functions while LSD does not have much effect on cognitive
abilities at bellow ego-dissolution dosages (though the psychedelic phenomena can be considered as a cognitive breakthrough, so this is debatable).
THC is also a strong sedative, while LSD and psilocibin are not. So I think you chose a totally wrong class of hallucinogens to compare. THC can
utmost be compared to some degree with NMDA blockers or even antimuscarincs.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
| Quote: |
I can't agree Nicodem ─ by itself, THC is quite mild by comparison to, say, LSD or psilocybin . . . Some (fast-disappearing) low-latitude
sativas are certainly hallucinogenic, but this is due to other relatively less investigated compounds such as THCV which potentiate THC's ability to
produce hallucination! I used to cultivate, years ago, sativas which would produce a 'trip' that would often be a helter skelter experience (sigh)!
|
Cannabidiol is an agonist for the 5HT1A receptor. A lot of Western-Indicas (type 2/3, wide leaf) have high cannabidiol and cannabinol. Traditionally,
they were used for hashish production in Northern India towards Pakistan, so they were not bred the same way as Type 1 (narrow leaf) Indicas and
certain Sativas. Sativa plants have a lot of genetic diversity (they were the original C. sativa species), so there is so much variation in the
cannabinoid partition.
But yea, THC elicits hallucinogenic effects in a different manner than psychedelics. It's more of a slowed release of neurotransmitters than actual
receptor agonism. Although the pathway is still complex and poorly understood.
|
|
|
DrEntheogen
Harmless

Posts: 18
Registered: 1-2-2012
Member Is Offline
Mood: Reacted
|
|
so mild hallucinogen could be considered correct, and ive deffinantley smoked enough, but im talking about actual visuals. and i joined this forum to
converse, learn more, and eventualy help other people learn. you realy dont need to be so aggresive about things, im not giving false information and
saying its true.
"Leonurine is one of the chemical constituents of the South African plant Leonotis leonurus. It is a mildly psychoactive alkaloid found in species
Leonotis nepetifolia, Leonotis artemisia as well as other plants of family Lamiaceae. Leonurine is easily extracted into water, as well as from the
essential oil of Leonurus sibiricus.[1" wikipedia.org
Okay mildly psychoactive, heres the ref. and ill make sure everything is referenced from now on.
and if you know so much which im sure you do, share your knowledge with me instead of trying to make me look like a fooll, i did nothing to you so
theirs no reason to be llike that.
"Orbitals are for mathematicians - Organic chemistry is for people who like to cook!" - Alexander Shulgin.
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
| Quote: | | Besides, the hallucinations caused by LSD and psilocibin are mainly visual while THC causes primarily spatio-temporal hallucinations and closed eye
visuals. |
Like when your hands become great mountains and you're looking down on them from a 'plane?
P
"I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones"
A Einstein
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
I have just begun to read Dr.Nichol's work. At any rate - finding the binding site geometry from the substrate is a daunting task. Stereochemistry,
rigidity, ortho-versus-para/meta substituents all play crucial roles. It was even proposed that identical compounds will have strongly different
effects depending on their orientation in the receptor (seems obvious). He had pronounced some work in 2011 that was deemed too dangerous to be
published - the activity of the compound he had created was so active in the brain he decided to keep the work unpublished, for fear of the black
market. He has been known to feel responsible for many deaths from the use of drugs he has synthesized.
I've also picked up a book in the library from here - fundamentals of medicinal chemistry or something of the like. Very interesting. The Topliss
graph is interesting as well, however I don't quite understand how they come up with it.
THC affects very different receptors, and its effects are to match - it is very hard to compare THC to LSD or DMT or Psilocybin/psilocin. Come to
think of it - LSD is one of the most active psychoactives in terms of how many receptors it interacts with, which is one of the reasons it is
"relateable" to most other compounds.
With that said, if you have ever had lack of sleep and smoked a great deal of THC I can assure you there is a good chance you will have a full blown
hallucinatory experience. I've had two. I sat in my room, while high, coming down, and very, very tired. My entire room became the moon, and the lunar
lander was right in front of me. Earth was in the distance. I had to focus for the image to stay, any eye movement quickly placed me back in my room.
I do not like THC very much. It's affinity to produce paranoia & delusional thoughts has steered me away from it. Its more medicinal effects
(primarily from the compound CBD & CBN) do help me sleep and become lethargic when I feel anxious.
It is interesting, to me, to have read this:
NN-DMT is one of the most potent hallucinogens known, and MDMA is one of the most potent euphoria drugs, acting on receptors that release dopamine,
etc. However, Methylenedioxy-nn-dimethyltryptamine has zero activity. Therefore, there is a very delicate issue of geometry, size, and sometimes even
more importantly, solubility in the BBB.
[Edited on 16-2-2012 by GreenD]
|
|
|
AirCowPeaCock
Hazard to Others
  
Posts: 311
Registered: 9-1-2012
Location: In your nation!
Member Is Offline
Mood: Hazardous
|
|
Quote: Originally posted by Nicodem  |
Do you mind describing its synthesis or at least provide the characterization data? You sure don't expect anyone is going to believe such
unsubstantiated claims. |
From wild-dagga (Leonotis leonurus), while its possible the effects produced were from another alkaloid--I doubt it.
BOLD
|
|
|
DrEntheogen
Harmless

Posts: 18
Registered: 1-2-2012
Member Is Offline
Mood: Reacted
|
|
Quote: Originally posted by GreenD  | I have just begun to read Dr.Nichol's work. At any rate - finding the binding site geometry from the substrate is a daunting task. Stereochemistry,
rigidity, ortho-versus-para/meta substituents all play crucial roles. It was even proposed that identical compounds will have strongly different
effects depending on their orientation in the receptor (seems obvious). He had pronounced some work in 2011 that was deemed too dangerous to be
published - the activity of the compound he had created was so active in the brain he decided to keep the work unpublished, for fear of the black
market. He has been known to feel responsible for many deaths from the use of drugs he has synthesized.
I've also picked up a book in the library from here - fundamentals of medicinal chemistry or something of the like. Very interesting. The Topliss
graph is interesting as well, however I don't quite understand how they come up with it.
THC affects very different receptors, and its effects are to match - it is very hard to compare THC to LSD or DMT or Psilocybin/psilocin. Come to
think of it - LSD is one of the most active psychoactives in terms of how many receptors it interacts with, which is one of the reasons it is
"relateable" to most other compounds.
With that said, if you have ever had lack of sleep and smoked a great deal of THC I can assure you there is a good chance you will have a full blown
hallucinatory experience. I've had two. I sat in my room, while high, coming down, and very, very tired. My entire room became the moon, and the lunar
lander was right in front of me. Earth was in the distance. I had to focus for the image to stay, any eye movement quickly placed me back in my room.
I do not like THC very much. It's affinity to produce paranoia & delusional thoughts has steered me away from it. Its more medicinal effects
(primarily from the compound CBD & CBN) do help me sleep and become lethargic when I feel anxious.
It is interesting, to me, to have read this:
NN-DMT is one of the most potent hallucinogens known, and MDMA is one of the most potent euphoria drugs, acting on receptors that release dopamine,
etc. However, Methylenedioxy-nn-dimethyltryptamine has zero activity. Therefore, there is a very delicate issue of geometry, size, and sometimes even
more importantly, solubility in the BBB.
[Edited on 16-2-2012 by GreenD] |
Wow, that is amazing about receptors and their being so much to consider, test, try, figure out. but its allso amazing knowing that we as humans are
so complex. id love to have had what happened to you smoking some herb in your room and completley hallucinating happen to me, ive smoked for 10 years
now and have never come close to that. im not doubting it i would just love to have an experience from marijuana similar to that.
Hallucinogens and empathogens/entactogens to me are for far more than to just feel good and have fun(even though that is a great part but you learn so
much about yourself and everything around you. its just incredible.
"Orbitals are for mathematicians - Organic chemistry is for people who like to cook!" - Alexander Shulgin.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DrEntheogen  | | so mild hallucinogen could be considered correct, and ive deffinantley smoked enough, but im talking about actual visuals. |
The thread title is "Ideas for new hallucinogens?". Hallucinogens induce hallucinations. Hallucinations are not only visual. Humans have several other
senses and perceptions as well. "Hallucinogens" is a term that encompass all kind of compounds that alter perception. Many type of compounds are
hallucinogens, the psychedelics are just one of these types and hallucinogenesis is not even the main thing that makes them interesting to most
people. The archetypal hallucinogens that fit the definition perfectly are the antimuscarinics, for example scopolamine. Leonurine certainly has
nothing to do with this topic.
| Quote: | | and i joined this forum to converse, learn more, and eventualy help other people learn. you realy dont need to be so aggresive about things, im not
giving false information and saying its true. |
I'm always straightforward, or what you call aggressive, when new members try to ignore the fact that this forum is a science forum (just like its
title implies). You might not perceive that you are giving false information, but you are. It is all a matter of your perception not being used to
scientific scrutiny. You are reproducing a wikipedia myth based on a false reference. Have you checked if the wikipedia entry on Leonurine (http://en.wikipedia.org/wiki/Leonurine) is true? Have you verified if its only reference, the article Experientia, 35, 571–572, truly
contains the information claimed in the wikipedia entry? Obviously you did not. If you would have read my previous replies with more attention you
would have realized by now, from the discourse I used, that I'm already aware of that bogus wikipedia entry and I specifically asked you for a
"veritable reference". Wikipedia is never a "veritable reference" until all its references are verified - it is an encyclopedia after all!
| Quote: | | and if you know so much which im sure you do, share your knowledge with me instead of trying to make me look like a fooll, i did nothing to you so
theirs no reason to be llike that. |
The best you can do to look like a fool is to open a referenceless thread and try to deviate the topic away from science on a science forum.
What kind of a reply is that? First you claim that leonurine "can induce vivid dreams at higher doses" and now you involve a totally different
material. How many times do I have to repeat that this is a scientific forum? You give no reference that Leonotis leonurus contains leonurine
and make some parabole that this plant material equals some compound which is only present in some related Lamiaceae species. Furthermore, the actual
plants that contain leonurine are not psychoactive (if they would be, it would be known as they are used as traditional medicine drugs). Besides, even
a cursory look at the molecular structure should have been enough to realize that it is an N-alkylguanidine, thus not in accordance to the claims of
Leonotis leonurus being smoked for attaining psychoactivity.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Dr.Entheogen, please look up psychonaut.com - you would fit right in with that community.
(Or Dmtnexus.org - they have some very, very intelligent people on that site!)
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
N-(substituted)benzyl compounds are the way of the future. How many more variations will have to be tried before the 0.3 mg active dose of 25I can be
surpassed? What are the potential results of easy to produce (and relatively user friendly) hallucinogens that cost a fraction of a cent per dose?
That reality has already arrived boys. Just in time...
Unlike LSD, NBOMe compounds can be made from extremely basic materials. How about optimizing an OTC synthesis rather than wasting your time trying to
compete with the PhD's using supercomputers to develop novel compounds?
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
extremely basic materials? where does one obtain brominated catechols?!
oh yeah chemistry.
still they don't look as easy as nn substituted tryptamines... decarboxylation, bromo-alkane, stir with some TEA or DIPEA... plus those nbome
coompounds have, from what I've seen, caused quite some damage. Not very user friendly - although who the fuck knows what people are actually getting
now days.
"I took some acid it lasted 48 hours and tasted bitter."
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
The most potent natural hallucinogen is Salvinorin A.
Found in Salvia divinorum is active in doses as low as 200 micrograms according to wikipedia. I am personally against the legalisation of marijuana,
but I think other hallucinogens should be made legal. There are so many hallucinogens in nature already, if you know which plants to pick and which
mushrooms to bring home. Regulations just favour the manufacture of hallucinogens in the lab with precursors that make your limbs fall off
(ergotamine).
I'm surprised that no one mentioned this hallucinogen before.
[edit]
I'm not sure why the picture is blacked out, but clicking on it displays the structural formula of this substance.
[Edited on 2-18-2012 by White Yeti]
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
No GreenD you are wrong. NBOMe compounds that I have experience with are definitely more user friendly than 2Cs and has a shorter duration. In fact,
so far they have achieved a better ratio of good reviews:bad reviews than most compounds. You're thinking of things like bromodragonfly, which is a
whole different ballgame. NBOMes are not the first superpotent phenethylamines, but they are the first ones to actually be usable. Their safety
profile has been shown to be far greater than things like bromodragonfly or DOI. Still can't match LSDs safety margin, so yes there is some potential
danger if dumbasses sell it as LSD. For now, I believe other compounds are far more commonly sold as "acid" simply because NBOMes are not widely
known/available yet.
Tryptamines are worth exploring for sure, but things that are 2 orders of magnitude more potent seem worthwhile too...
Yes, salvinorin is quite potent... but that's about all it has going for it. It's effects were surely quite impressive, but not something people often
want to repeat. And calling it a hallucinogen is a little generous, dissociative would be more appropriate in my experience.
[Edited on 18-2-2012 by 497]
[Edited on 18-2-2012 by 497]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 497  | | Yes, salvinorin is quite potent... but that's about all it has going for it. It's effects were surely quite impressive, but not something people often
want to repeat. And calling it a hallucinogen is a little generous, dissociative would be more appropriate in my experience. |
Seems like nobody here actually knows or checked what the term "hallucinogen" means. As this misinterpretation keeps on going on here even after I
explained it already, I will go as far as to cite the dictionary:
| Quote: |
hal·lu·ci·no·gen
[huh-loo-suh-nuh-juhn]
noun
a substance that produces hallucinations.
hal·lu·ci·na·tion
[huh-loo-suh-ney-shuhn]
noun
1. a sensory experience of something that does not exist outside the mind, caused by various physical and mental disorders, or by reaction to certain
toxic substances, and usually manifested as visual or auditory images.
2. the sensation caused by a hallucinatory condition or the object or scene visualized.
3. a false notion, belief, or impression; illusion; delusion.
|
As you can see, dissociatives fit the definition perfectly. Actually, dissociatives, either NMDA blockers or kappa receptor antagonists, and also
cannabinoids and antimuscarinics are more aligned with the above definition than psychedelics, especially in consideration to the the point 3 of the
definition of the term "hallucination".
It is not really that common to have realistic hallucinations encompassing also delusions on psychedelics even at the doses where the effects are
already heavily "manifested as visual or auditory images". Of course, the interpretation of the experience can lead one into accepting some
perceptions and impression as more or less realistic, but this depends more on the individual neurotic background rather than the effect of the drug
on the mind. Even though delusions can be achieved on psychedelics at above ego-dissolution levels, they are not really a typical component of the
psychedelic phenomenon.
On the other hand, those that have experience with scopolamine and similar compounds know very well what a real hallucinogen is. Even at mild doses of
antimuscarinics, delusions are inevitable and what is "manifested as visual or auditory images" is often indiscernible from reality, at which point
reality becomes a joke you play to yourself. LSD is a walk in the park in comparison to scopolamine when it comes to which is a more efficient
hallucinogen. When it comes to the real stuff, you will not be talking about aesthetically beautiful kaleidoscopic images and arabesques, like you
would be talking about some nice psychedelic. With scopolamine it is a nightmare come true. A correct dose salvinorine is like a disintegration into
atoms. A heavy dose of THC can be madness and paranoia at its best. Psychedelics most certainly are hallucinogens, but they pale in comparison to the
more effective ones.
This thread is becoming desperately useless and is still more or less referenceless, so to bring it back to science I have actually taken the time to
check the literature in regard to Leonotis leonorum. The plant has been quite well studied in regard to its constituents, but as far as I
could find, the psychoactive constituent is not yet known. The aerial plant material is rich in labdane diterpenoids. The major diterpenoid
constituent is marrubiin which is otherwise better known as a constituent of Marrubium vulgare. Two new labdanes have been determined just
recently, 9,13-epoxy-6-hydroxylabdan-16,15-olide and 9,13:15,16-diepoxy-6,16-labdanediol (Biochemical Systematics and Ecology 2011, 39,
216-219). Long chain terpenoids have also been isolated, such as 1,2,3-trihydroxy-3,7,11,15-tetramethylhexadecan-1-yl-palmitate and others
(Phytochemistry Letters 2009, 2, 103-105). Some flavonoids have also been found in aerial parts of the plant (Pharmaceutical Biology
2009, 47, 894-902). The review on the furan-diterpenoids from the genera Leonotis and Leonurus (Heterocycles (2007), 74, 31-52) covers the
references up to the year 2006. Bellow is the graphical excerpt of the structures of some diterpenoids in L. leonorum. These compounds are
structurally related to salvinorins, but don't get mislead by this - affinity to the kappa receptors is extremely sensitive to structural
modifications of salvinorin A, and thus structural similarity mean absolutely nothing.
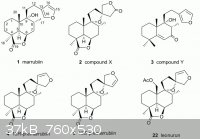
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
| Pages:
1
2
3 |