bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
'Polyethylene Glycol 3350 NF'
I recently underwent a laparoscopic appendectomy and was given a bottle polyethylene glycol (PEG) with an average molecular weight of 3350. After
finishing with the prescribed regimen of painkiller and PEG laxative, I have half a bottle (maybe 450 grams) of the PEG remaining. Besides ingestion
and combustion, does anyone have a suggestion of something <em>interesting or useful</em> to do with it? A forum search didn't turn up
much.
|
|
|
zoombafu
Hazard to Others
  
Posts: 255
Registered: 21-11-2011
Location: U.S.
Member Is Offline
Mood: sciencey
|
|
I suppose as a sexual lubricant...
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
It can be nitrated if you have an interest in "energetics"...
correction: mistake, I was thinking of polyvinyl alcohol
[Edited on 8-2-2012 by AndersHoveland]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
That would do just about nothing, seeing as how the only available sites to add nitro esters are on the ends of the obscenely long chains.
Sounds painful, as it's a powder.
I'd treat it as a non-volatile polar solvent for high-temperature reactions.
[Edited on 2-7-12 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I use PEG 1500 as the medium for my oil bath. It solidifies at room temperature (mp. 44-48°C), so there's no danger of spills when the oil bath is
not in use, and most importantly, the solidified PEG is very water soluble so I can simply rinse the flasks with water and they become perfectly clean
on the outside. No more greasy mess as with other oils or fats!
The solid PEG1500 is not noticeably hygroscopic, its surface stays dry and waxy.
The principal disadvantages of this otherwise perfect heat carrier are the price (ca. EUR 17 per kg), the high viscosity of the liquid below 100°C
(it becomes less viscous when hotter)
and the mediocre stability towards heat and oxygen above 150°C. An antioxidant needs to be added (ideally, phenothiazine or
phenyl-alpha-naphthylamine) in order to make the bath stable for prolonged operation above 150°C.
See page 15, right side:
http://www.essentialingredients.com/pdf/PolyglykolsforPerson...
However, someone I know has used this bath in the lab for many months without stabilizer, and the only symptoms were darkening and slow "evaporation"
of the liquid. The bath remains useable despite the oxidation and weight loss, and I still think that PEGs are near perfect oil baths for the lab when
temperatures are not overly high.
|
|
|
Organikum
resurrected
    
Posts: 2344
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Phase transfer catalyst in organic reactions, benzylalcohol to benzaldehyde for example, just to name a basic one.
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
According to Wikipedia, PEG is an anti-foaming agent used in some foods. Perhaps it could prevent foaming in a reaction? I have a bottle of the same
stuff from a colonoscopy a few years back but I've yet to find a use for it either.
Victor Grignard is a methylated spirit.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I now have a few hundred grams of polyethylene glycol 3350 sold as a laxative. I'm wondering also how I might put it to a higher use. Here's its
formula:
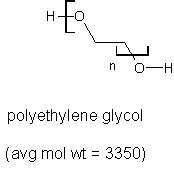
What I would really like to do is depolymerize it down to glyme or diglyme, both expensive, hard-to-find, and useful solvents:
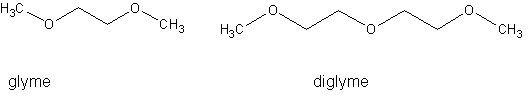
Does anyone know how to do this?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I don't think that you can make glyme from this easily. Forming the methyl ether of a primary alcohol is normally done via Williamson synthesis with
alkyl halide or dialkyl sulfate.
Glymes are made from ethylene oxide and dimethyl ether, this way the methyl ether bonds are formed via ring opening of the oxirane.
You could try converting it into dioxane. That would be very useful too. Dioxane can be used for coalescence and purification of dirty potassium.
Try distilling some of the PEG slowly over a free flame with a small amount of sulfuric acid added. Do you know how dioxane smells like?
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  | | I now have a few hundred grams of polyethylene glycol 3350 sold as a laxative. I'm wondering also how I might put it to a higher use.
|
Hair moisturizer? It's certainly, uh, higher.
Sorry, that was too good a straight line you provided.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by garage chemist  |
Try distilling some of the PEG slowly over a free flame with a small amount of sulfuric acid added. Do you know how dioxane smells like?
|
I will give this a try. No, I don't think I've ever used dioxane.
UnintentinalChaos has a nice YouTube video showing how he depolymerizes polystyrene. If only it were that simple with PEG.
Yes, the poor PEG is surely begging for a use with a little more dignity. 
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
You can also catalyze the hydrolysis to dioxane: see US patent 4283339 ...
The less you bet, the more you lose when you win.
|
|
|
Morgan
International Hazard
    
Posts: 1750
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Bubbles perhaps.
"J-Lube may be the most important polymer for creating giant bubbles. Less than a gram of this powder can turn a gallon of water and twelve ounces (or
less) of dishwashing liquid into a potent giant bubble juice. A 10 oz. bottle of J-Lube powder costs less than $20 (including shipping) and can make
hundreds of gallons of bubble juice."
"J-Lube is a powder made up of 25% Polyethylene Glycol (often called PEO since it is also known as polyethylene oxide) which is the active ingredient
and 75% sucrose which essentially acts as a dispersant. Its primary commercial use is for aiding veterinarians in the birthing of livestock. It adds
valuable self-healing qualities to bubble solutions which enables bubbles to complete themselves and enables bubble-in-bubbles. It also reduces the
likelihood that a bubble will tear coming off of the wand. In large quantities, it can make bubbles so self healing that any disturbance of the air
will break up a large bubble into smaller bubbles. It is thought to work very well with the cellulose-based compounds like Hydroxypropyl
Methylcellulose (HPMC) (see above -- it is found in Surgilube) and' 'Hydroxyethyl Cellulose (HEC) (see above -- it is found in KY-Jelly and its
knockoffs). Many bubblers report synergies betwen these compounds so that solutions that include J-Lube and either HPMC or HEC have properties that
exceed those of solutions with only of these ingredients."
http://soapbubble.wikia.com/wiki/Ingredients
http://en.wikipedia.org/wiki/Polyethylene_glycol
Big Outdoor Soap Bubbles ~ Recipe & How To Do. Time Warp Discovery Channel Featured Expert
http://www.youtube.com/watch?v=gQmpVIgDvgQ
Keith Michael Johnson explores how bubbles work & what we can do with them. BUBBLEOLOGY
http://www.youtube.com/watch?v=8_5AsgMM4CE&list=UU6WtmFF...
|
|
|