GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Hate this stuff
Phosgene
Chloroplast / phyll
Ammonium hydroxide (see other thread)
Chrome alum
Why do they do this?!
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
(er) What? ( ) )
P
"I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones"
A Einstein
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
ITHM that phosgene does not contain phosphorus, chlorophyll no chlorine, chrome alum no aluminium, urine no uranium, et cetera, et cetera.
Ammonium hydroxide does not fit the list, since it formally does behave like a solution of the hydroxide salt of ammonium.
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
You're good! That's what you are! Good! ( ) )
P
"I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones"
A Einstein
|
|
|
Vikascoder
Hazard to Others
  
Posts: 309
Registered: 28-1-2012
Member Is Offline
Mood: No Mood
|
|
Ya this information is correct but why ammonium hydroxide is to be hated
|
|
|
Pulverulescent
National Hazard
   
Posts: 793
Registered: 31-1-2008
Member Is Offline
Mood: Torn between two monikers ─ "hissingnoise" and the present incarnation!
|
|
'Cos it stinks? ( ) )
P
"I know not with what weapons World War III will be fought, but World War IV will be fought with sticks and stones"
A Einstein
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
it stinks, and there "is no such thing as ammonium hydroxide" !
There is ammonium
There is hydroxide
There is no ammonium hydroxide
any other examples of this? Urine and uranium I think is a stretch haha
[Edited on 7-2-2012 by GreenD]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
There is no point in examples since ALL of chemistry is like that. One reason is that many trivial names were coined when comprehension of the
structure of matter was not very deep (e.g. carbohydrate, chloral hydrate), but.more importantly, because chemistry is about CONCEPTS. We don't care
what it really looks like - what matters is how it reacts and how you can describe it. Think about the huge field of hydrous silicate and aluminate
phases. Of course Al2O3.xH2O and SiO2.xH2O do not exist in that form - but describing them that way is very useful from a conceptual point of view.
Likewise hypophosphorous acid may not exist (it's actually phosphinic acid), but both give rise to different, isolable derivates (esters). Or think
about the synthons in organic chemistry. In the same vein, an ammonium hydroxide solution makes perfectly sense: if you add an acid it reacts like a
solution of ... well ... ammonium hydroxide.
Edit: Oh, and some of the examples you gave simply come from the same root: chloros=green (obvious), phos=light (white phosphorus emits light,
phosgene is created with light). That's where some general education helps. 
[Edited on 7-2-2012 by turd]
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Ammonium hydroxide is real. Ammonia is basic, and gets protonated by water, leaving a hydroxide ion for each ammonium ion.
I weep at the sight of flaming acetic anhydride.
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Quote: Originally posted by turd  |
Edit: Oh, and some of the examples you gave simply come from the same root: chloros=green (obvious), phos=light (white phosphorus emits light,
phosgene is created with light). That's where some general education helps. 
[Edited on 7-2-2012 by turd] |
I actually realized the chloro after posting. I hadn't known that phos = light. And bringing up carbohydrates - I now realize how prevalent this
misleading nomenclature is!
|
|
|
DougTheMapper
Hazard to Others
  
Posts: 145
Registered: 20-7-2008
Location: Michigan, USA
Member Is Offline
Mood: Energetic
|
|
A "phosphor" is a broad term describing a substance which will "fluoresce."
Most phosphors do not contain phosphor, and most fluorescent compounds do not contain fluorine. I believe phosphorus was named with the prefix "phos"
because when it was first discovered it glowed (burned) in air. As for fluorescence, this term was derived from the phenomenon of glowing fluorite
crystals because of a europium impurity and was originally thought to be the fluorite itself.
Victor Grignard is a methylated spirit.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
How about theobromine, a xanthine derivative that contains no bromine.
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
In Analytic Chemistry class, we got complex equilibria beat into our
thick hormone-sodden undergraduate skulls, so excuse me if I prattle on.
Room temp pKb for NH<sub>3</sub> is 4.75 which corresponds to 0.4% dissociation before you include activity coefficients.
This suggests there's a lot more NH<sub>3</sub>(aq) than NH<sub>4</sub>OH. Suggests? Like Hell! It's an irreducible fact.
RT solubility in water is ~30%, = ~18M, = 1:2 molar ratio of NH<sub>3</sub> to H<sub>2</sub>O.
This means that any honest model of aqueous ammonia needs a coupla waters in the picture.
This empirical force field model tries hard to show the hydrogen bonding.
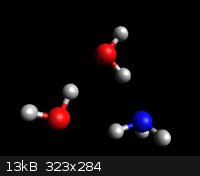
Ab initio modeling twists the water mols ~10 degrees out of co-planarity.
That's all for now.
|
|
|
arsphenamine
Hazard to Others
  
Posts: 236
Registered: 12-8-2010
Location: I smell horses, Maryland, USA
Member Is Offline
Mood: No Mood
|
|
It's all Greek to me.
|
|
|
thesourcerer
Harmless

Posts: 6
Registered: 4-3-2012
Member Is Offline
Mood: No Mood
|
|
I don't see any problem with Bromine and Theobromine. Theobromine is an alkaloid from the species Theobroma cacao, and it is usual nomenclature to
name an alkaloid from the plant and end in ine. The species Theobroma was named long before Bromine was discovered. They are both derived from the
greek Bromos (stench), whilst Theo means god. Well I think cocoa (or chocolate) has a godly smell, but Bromine definitely stinks. cf Chlorophyll and
Chlorine, both green and hence the names, but why should that insinuate any chemical connection?
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Bromine doesn't stink. Its scent it similar to chlorine and iodine, and if concentrated low enough, reminds of photographic development stores.
"Stench" is something hydrogen sulphide and impure carbon disulphide are described with. Not simple oxidizers that smell of disinfectants.
|
|
|
Magic Muzzlet
Hazard to Others
  
Posts: 146
Registered: 22-7-2010
Member Is Offline
Mood: No Mood
|
|
????
But then again I guess using a flame on glassware isn't classic either right.
You have either ruined your noses ability to smell or never used pure liquid Br2 because I cannot fathom how you can make that comment. Unless you are
joking.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
"rhodanide" has nothing to do with rhodium.
A befriended dietician says he gets uncountable numbers of patients that talk about their 'chloresterol' levels. I guess the 'chlor' makes it sounds
more nasty than the correct spelling.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
When Mr. A.G. Lee sat down and wrote his work "The Chemistry of Thallium" he picked two very illustrative quotes that relate exactly to the problem at
hand. These I have committed to memory:
| Quote: | | So now we can nail down these changeable humors and tell them to their faces what species and class they belong to. That is man's prerogative on
earth: to call things by name and put them in a system. They cast down their eyes before him when he calls them by name, for to name is to command."
- Thomas Mann |
That quote contrasts beautifully with its companion:
| Quote: | | Wherever primitive man put up a word, he believed he had made a discovery. How utterly mistaken he really was! He had touched a problem, and while
supposing he had solved it, he had created an obstacle to its solution. Now, with every new knowledge we stumble over flint-like and petrified words
and, in so doing, break a leg sooner than a word. - Nietzsche |
|
|
|
ScienceSquirrel
|
Thread Pruned
13-3-2012 at 05:32 |