gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Nitrating TCCA with AgNO2
Hello, I've been reading the forum recently and found interesting information about nitrating tcco with silver nitrite. I presume that the reaction
looks like this:
C3N3O3Cl3+3AgNO2---->C3N3O3(NO2)3 + 3AgCl
Also when I had been googling some examples of nitration with silver nitrate I found that this reaction takes place at room temp. and is complete
after several hours.
Has somebody tried this reaction except for the guy that mentioned it on this forum( unfortunaly he was on this forum last time in 2002)?
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
As far as I know, only NO2+ ions can nitrate substances. Both the Cl- in TCCA and the NO2- in AgNO2 are anions. So I think you would get NO2- ions in
the cyanuric acid. I don't think NO2- can nitrate the cyanuric acid.
I could be wrong, though.
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
http://www.organic-chemistry.org/synthesis/C1N/nitro-compoun...
Look at first reaction, although they say it is possible for bromine and iodine to be replaced
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
I see two problems:
.AgNO2 can nitrate alkanes, but that does not mean it can nitrate a carboxylic acid.
.Cl- might be too electronegative for the NO2- to replace (nitrate)
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Yes, that's right, but one guy on this forum said that this is possible.
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/viewthread.php?action=pri...
Post by Nick
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
What is exactly "nitrating" in this context? Nitrating to my opinion is the action of replacing a group (usually a -OH group) by a nitrate group,
-O-NO2. The classic example is making glyceryl trinitrate from glycerol. This compound often incorrectly is called nitroglycerine.
The things described in this thread are replacements of chlorine by -NO2 (or -O-NO). I would not call them nitrations.
Also keep in mind that it makes a big difference whether you have -NO2 or -O-NO. A ncie example is the difference between nitromethane (CH3-NO2),
which is a colorless liquid, boiling just over 100 C and methylnitrite (CH3-O-NO), which is a colorless gas (and is easily made by adding an aqueous
solution of NaNO2 to an acidified solution of CH3OH in water).
I do not expect that TCCA can be easily made into a nitro compound or a nitrite compound with AgNO2. TCCA is strongly oxidizing and I expect that it
will oxidize nitrite ion to nitrate ion at once, in the process releasing chloride ion, which will precipitate AgCl.
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Hmm. So what would the overall reaction be? How is it going to oxidize nitrite to nitrate?
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
And why would they(organic-chemistry.org) say, that silver nitrite makes nitro compounds?
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
 
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
And would it be possible to replace chlorines with -OH groups and then replace Hydrogens in -OHs with nitro groups?
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
I guess it would be something like this: C3N3O3Cl3+3AgNO2====>3AgCl+C3N3(NO3)3. You see, it is not a nitration reaction.
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
But chloroalkanes have different structures. So maybe it might work for them.
[Edited on 30-1-2012 by weiming1998]
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Write TCCA as R(OCl)3 for convenience. the R-group does not change, this is the C-N trimer/ring.
What most likely happens now is the following:
R(OCl)3 + 3H2O + 3AgNO2 ---> R(OH)3 + 3AgCl + 3NO3(+) + 3H(+)
Here, R(OH)3 is cyanuric acid and R(OCl)3 is TCCA in one of its tautomeric forms. The TCCA is in equilibrium with hypochlorous acid and cyanuric acid.
Hypochlorous acid is a VERY strong oxidizer which easily oxidizes nitrite.
| Quote: | | nd why would they(organic-chemistry.org) say, that silver nitrite makes nitro compounds? |
Sometimes nitrites
make nitro compounds, sometimes they do not make nitro compounds. It depends on what is treated with the AgNO2. But this is not a nitration, nitration
is adding nitrate to compounds, adding nitro to compounds is a different thing. Because of the strongly oxidizing nature of TCCA I do not expect
formation of any nitro compound, but simply oxidation of nitrite to nitrate where the nitrate goes into solution as NO3(-).
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Ok, thank you for your help 
Then I have another question, is it possible to add (-NO2) groups to cyanuric acid using nitric acid?
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
Quote: Originally posted by gl92038  | Ok, thank you for your help 
Then I have another question, is it possible to add (-NO2) groups to cyanuric acid using nitric acid? |
I don't think you can, because they are both acids. Also, Nitric acid gives NO3- and H+. If it gives NO2-, the nitrogen has to be reduced from +5 to
+3 in the oxidation state, which is highly improbable because:
.As far as I know, cyanuric acid is not that powerful as a reducer
.The NO2- specimen in acidic solutions is extremely unstable
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
But phenol is also acidic(pKa= 10, not as much as cyanuric acid pKa= 7, but still) and its derivative trinitrophenol is stable.
Is there a slightest chance of making this compound
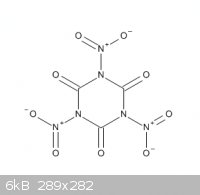
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
Quote: Originally posted by gl92038  | But phenol is also acidic(pKa= 10, not as much as cyanuric acid pKa= 7, but still) and its derivative trinitrophenol is stable.
Is there a slightest chance of making this compound |
Phenol is first sulfonated with concentrated sulfuric acid, which turns into phenosulfonic acid, then nitric acid is induced. As nitric acid can act
as a base and give out NO2+ ions in the presence of an acid and a catalyst (usually sulfuric acid), the NO2+ group exchages with the sulfonic acid
group, which probably acts as the acid that causes nitric acid to release NO2+. There is no involvement of NO2-, which probably would have decomposed
in such strong acid solutions.
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
Perhaps with a sulfuric acid catalyst+ nitric acid, you can make nitrocyanuric acid. But cyanuric acid by itself is too weak as an acid for the nitric
acid to release the NO2+ ions.
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Thank you 
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Btw, has somebody tried this reaction, I mean nitration of cyanuric acid with the mixture of sulfuric and nitric acids?
|
|
|
weiming1998
National Hazard
   
Posts: 616
Registered: 13-1-2012
Location: Western Australia
Member Is Offline
Mood: Amphoteric
|
|
I don't think so, but it'll be worth a try with a mix of conc. sulfuric acid and nitric acid.
|
|
|
gl92038
Harmless

Posts: 31
Registered: 14-1-2012
Member Is Offline
Mood: No Mood
|
|
Ok, I'll try it someday 
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I do not expect formation of the structure you have drawn, with three nitro groups attached to the C/N ring. If any nitration occurs with conc. H2SO4
and HNO3, then I expect -O-NO2 groups attached to the ring instead of -NO2 groups. But still, I have doubts, because of the N-O-N structure in such a
compound, but I do not dismiss the idea beforehand. You could give it a try with cyanuric acid as starting compound. Do not use TCCA as starting
compound, that definately will not work.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
This thread is terrible, but to stop this idle and referenceless discussion, I can let you all know that N,N',N''-trinitrocyanuric acid is not a known
compound. There are only 6 references mentioning it, and 5 of them are about theoretical calculations. It is mentioned as a real-world compound only
in one reference, which is a patent application where this compound is used only as a virtual diversion to widen the claims beyond the keto-RDX
example (US6139054). Even theoretical calculations end up with the conclusion that it is "absolutely unstable and hardly exist in a free state" (DOI:
10.1002/prep.200700040).
As far as I know, the N-nitration of imides has only been described using N2O5. After a long reaction time the nitroimides are obtained (see DOI:
10.1002/prac.19620150310 and the excerpt with the typical procedure bellow). Nitroimides are very sensitive to nucleophilic attack and thus hydrolyze
rapidly.

…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|