kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
p-benzoquinone+acetone condensation
It is surprisingly hard to find any informations about aldol condensation of p-benzoquinone with ketones.
As far as I remember, o-benzoquinone reacts with acetone giving normal product:
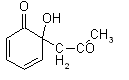
What about p- isomer ?
Does it give any isolable product(s) from such a condensation ?
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
very interesting I would have thought the fact that the oxygens are connected directly to the aromatic
ring would make it impossible to undergo aldol.
from the pic you have draw it looks like you loose aromatisity dure in condensation.
I wonder if its easy to regain this and aquire a phenylacetone after condensation.
maby a dehydration with persulfate might do it or would we require something with a stronger
dehydrating nature than this ?
e3500 console login: root
bash-2.05#
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kmno4  | | It is surprisingly hard to find any informations about aldol condensation of p-benzoquinone with ketones. |
p-Benzoquinones react with enolates of 1,3-dicarbonyl compounds or related C-nucleophiles to give either the corresponding 5-hydroxybenzofurane
product (with ketones) or a normal Michael adduct (with esters it can give the corresponding lactone as the end product).
For example, the formation of 3-acetyl-5-hydroxybenzofurane by the addition of acetylacetone on p-benzoquinone is described in Tetrahedron Letters
51, 2136–2140 (DOI: 10.1016/j.tetlet.2010.02.066).
In regard to enolate reactions with quinones, see also the Nenitzescu reaction which gives 5-hydroxyindoles from p-benzoquinones.
The Michael addition of diethyl malonate on p-benzoquinone was already discussed on this forum.
I found nothing on any possible reaction between acetone and p-benzoquinone with SciFinder. If there would be a reaction under basic conditions, I
would guess that 5-hydroxy-2-methylbenzofurane would be the expected product. I would guess the reason why this apparently does not happen is because
benzoquinone is unlikely to be stable toward the strong bases required for the enolization of acetone (such as hydroxides which can react with
quinones themselves).
| Quote: | | As far as I remember, o-benzoquinone reacts with acetone giving normal product: |
What is the reference? I found nothing on that compound. It is not listed in the Chemical Abstracts.
Quote: Originally posted by Ephoton  | very interesting I would have thought the fact that the oxygens are connected directly to the aromatic
ring would make it impossible to undergo aldol.
from the pic you have draw it looks like you loose aromatisity dure in condensation. |
He is talking about the reactions on p-benzoquinone which has no aromatic system. It can not lose any aromaticity, as there is none in the first
place. Why do you think quinones are such good oxidants? Exactly because they have a deep thermodynamic fall down to the corresponding aromatic
hydroquinone/catechol form.
| Quote: | maby a dehydration with persulfate might do it or would we require something with a stronger
dehydrating nature than this ? |
Persulfates are oxidants. I never heard of them being used as dehydrating reagents.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Nicodem - unfortunately I cannot find this article  
I have wasted few hours searching it. All I remember that it was something about synthesis of some heterocycle and this compound was part of synthetic
path. Authors tried to make some oxime from this compound and next to make cyclization...
Article was from ACS or Elsevier database, older than from 2000 year - that is all.
However I agree - this ketol looks suspiciously familiar, that is why I remembered it.
The chemistry of the quinonoid compounds (Patai,1974) gives some explanation to my questions. It is almost the same what Nicodem wrote -
instability of quinones in basic media.
It means 70% of tars and 30% of mixture of various products.
Such reaction (p-quinone+acetone) is so simple to do, that I was very surprised in not finding any synthetic reports about it.
But who wants to report tars and undefined products....
[Edited on 22-12-2011 by kmno4]
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
| Quote: | | As far as I remember, o-benzoquinone reacts with acetone giving normal product: |
What is the reference? I found nothing on that compound. It is not listed in the Chemical Abstracts. |
See the next link: Acta Chem. Scand. 14 (1960), 1643-1653,
http://actachemscand.dk/pdf/acta_vol_14_p1643-1653.pdf
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
I have found a document where 2,6-dimethoxy-quinone with acetone gives similar 1,2-addition product (1:1). It is also claimed that unsubstituted
p-benzoquinone and other simple quinones do not give such 1,2-addition products with acetone.
See the next link:
http://repository.ias.ac.in/68299/1/68299.pdf
|
|
|
kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Thanks for the informations.
From time to time, I still think about this reaction, ha.
I attach the publication (by Rao) in slightly better quality (from Springer), and reference no. 1 from it.
Attachment: BF03052734.pdf (130kB)
This file has been downloaded 466 times
Attachment: BF03171012.pdf (253kB)
This file has been downloaded 445 times
Слава Україні !
Героям слава !
|
|
|