franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Nitrocarbons
About time there was a thread devoted to nitrocarbons
On a speculative note I propose an unknown one derived from :
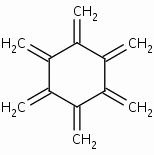
Hexamethylenecyclohexane also known as Radialene , CAS 3227-93-8
www.chemspider.com/Chemical-Structure.14476692.html
Reaction scheme :
C- nitration of all 12 hydrogen sites might be done under extreme conditions.
The stoichiometric amount of SO3 gas introduced steadily at some pressure
into a closed vessel , dissolves into a chilled mixture under it of fuming nitric
acid and radialene. As the water is abstracted the HNO3 in the mixture will be
consumed and replaced entirely with H2SO4 mixed with the end product
Dodecanitroradialene.
C6(CH2)6 + 12 HNO3 + 12 SO3 => 12 H2SO4 + C6(C{NO2}2)6
C6(C{NO2}2)6 => 12 CO2 + 6 N2
- comments ?
Related thread elsewhere
www.sciencemadness.org/talk/viewthread.php?tid=1226#pid17906...
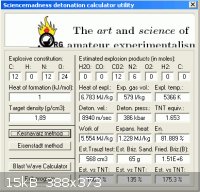
An empirical method to estimate detonation velocity described
on page 6 here -> Explosive Properties of Polynitroaromatics
http://handle.dtic.mil/100.2/ADA229627 , gives a value of 5.17
for factor F which works out to ~ 8930 m/s. The utility created by
forum member engager , utilizing the methodology of Keshavarz
obtains near to the same value ( 8940 ) and 386 kbar at a nominal density
of 1.89. The method I explained in these other posts
http://www.sciencemadness.org/talk/viewthread.php?tid=11195#...
http://www.sciencemadness.org/talk/viewthread.php?tid=11195&...
is in close agreement with detonation velocity of 8905 and 363 Kbar
at the supposed density of 1.9.
.
[Edited on 15-12-2011 by franklyn]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | Decanitroradialene. |
Wouldn't that be dodecanitroradialene?
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
The question is: Where can you get radialene?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
You might be interested in "Nitrocarbons", by Arnold T. Nielsen.
http://www.amazon.com/Nitrocarbons-Organic-Chemistry-Arnold-...
If you want a cheaper copy, that may or may be a cheap counterfeit in violation of international copyright laws...
http://www.flipkart.com/books/1560816813 (the price is equivalent to 140USD )
Also see the thread in this forum, "Hexanitroethane", C2(NO2)6,
http://www.sciencemadness.org/talk/viewthread.php?tid=6898#p...
[Edited on 15-12-2011 by AndersHoveland]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
An even better variant of the reaction would be the condensation product between CH2(NO2)2 and 1,2,3,4,5,6-cyclohexanhexone...
(C=O)6 + 6CH2(NO2)2 --> (C=C(NO2)2)6 + 6H2O
Thus a radialene with all H atoms replaced by NO2...C12N12O24...yeah perfect OB!
I suspect it will display a higher density than what you expected (based on the densities of tetranitronaphtalene, hexanitrobenzene and the relatives
densities between benzene, naphtalene, anthracene, ...)
Also radialene must be quite instable and prompt to rearrange into a central aromatic ring and 3 cyclobutane rings between C1 and C2, C3 and C4 and
between C5 and C6.
The very same may happen with our putative dodecanitroradialene??
[Edited on 23-1-2012 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I do not think it can condense like that.
dinitromethane can however condense with aldehydes, but this typically forms nitro-alcohols.
If by "1,2,3,4,5,6-cyclohexanhexone", you mean a compound with the formula C6O6, it is doubtful that this exists. The closest type of compound to this
that I can think of is squaric acid .
[Edited on 24-1-2012 by AndersHoveland]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Cyclohexanehexone Triquinoyl references
@ AndersHoveland
These three posts are in a series
http://www.sciencemadness.org/talk/viewthread.php?tid=9424&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=9424&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=9424&a...
.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Triquinoyl ( cyclohexanehexone )
J. Org. Chem.; 1986; 51(26); 5241-5243
J. Lerch, Ann. 124, 34 (1862)
R. Nietzki and T. Benckiser, Ber. 18, 499 (1885)
R. Nietzki and T. Benckiser, Ber. 18, 1833 (1885)
R. Nietzki and F. Kehrman, Ber. 20, 322 (1887)
F. Henle, Ann. 350, 330 (1906)
F. Bergel, Ber. 62, 490 (1929)
B. Eistert and G. Bock, Angew. Chem. 70, 595 (1958)
B. Eistert, G. Bock, E. Kosch, and F. Spalink, Chem. Ber. 93, 1451 (1960)
Triquinoyl octahydrate C6O6 8H2O
forms microscopic needles, melting at 95º C, evolving CO2.
Insoluble in cold water, alchohol and ether.
|
This is very surprising to me.
Why does C6O6 not spontaneously decompose to carbon monoxide? Does the water somehow stabilise it? Does anhydrous C6O6 exist? Is there any way to
polymerise carbon monoxide into cyclohexanehexone? Could a linear polymer of CO exist?
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Several of the nitrocarbons can themselves act as nitrating agents. Tetranitromethane, for example, reacts at room temperature with phenol to form
nitrophenol and trinitromethane in 100% yield. (at 30degC in water). At higher pH, nitrite is also results.
"Reaction of Tetranitromethane. I. Kinetics and Mechanism of Nitration of Phenols by Tetranitromethane."
Thomas C. Bruice, Maurics J. Gregory, Saundra L. Walters
Warning:
Both tetranitromethane and trinitromethane can form dangerously sensitive explosive mixtures with fuels. Mixtures of trinitromethane with hydrocarbon
fuels quickly decompose above 100degC, and the decomposition can lead to detonation.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
More on polymeric CO
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
An interesting caged molecule reminiscent of Cubane
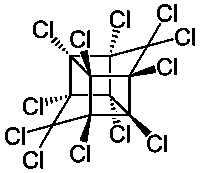
C10Cl12 Mirex CAS-2385-85-5 - Now banned as a persistent environmental
contaminant formerly used as an insecticide especially against fire ants, applied
under the original name Dechlorane of the defunct Hooker chemical co. as a
fire retardant for plastics, rubber, paint, paper, electric goods. Produced by the
dimerization of hexachlorocyclopentadiene in the presence of AlCl3. Known as
Hexachlorocyclopentadiene dimer , http://en.wikipedia.org/wiki/Hexachlorocyclopentadiene
http://www.chemicalbook.com/ChemicalProductProperty_EN_CB343...
also as Perchloropentacyclodecane , Dodecachloropentacyclodecane , IUPAC _
1,2,3,4,5,5,6,7,8,9,10,10-Dodecachloropentacyclo[5.3.0.02,6.03,9.04,8]decane
Density : 2.25 g/cm3
Sublimes : 421 °C at 1 atm.
Melts Decomposing above 485 °C
Insoluble in water , 0.085g/100ml at 20 ºC
Solubility : Chloroform 17g/100ml , Dioxane 15g/100ml , Xylene 14g/100ml
Benzene 12g/100ml , CCl4 7g/100ml , MEK 6g/100ml
http://en.wikipedia.org/wiki/Mirex
http://chemeo.com/cid/21-111-4
http://sitem.herts.ac.uk/aeru/iupac/1294.htm
http://www.chemspider.com/Chemical-Structure.16054.html
Now only available as a laboratory reagent it is very expensive. ~ $ 25 gm
http://www.carbosynth.com/carbosynth/website.nsf/(w-productdisplay)/47CC86BCE4D50450802579CF0063B54A
http://www.ebiochem.com/product/mirex-dechlorane-4070-10mg-vial-109359#morespecification
The precursor Hexachlorocyclopentadiene as well as Aluminum Chloride
are available as industrial staple chemicals. According to Skylighter
www.skylighter.com/fireworks/making-fireworks-projects/chlor...
it still finds use as a chlorine donor in pyrotechnics.
________________________________________________
The possibility of substituting the chlorine with nitro groups cannot be lightly
dismissed , given the prospective density for the target and positive oxygen
balance.
Nitration with nitrates was pioneered by Menke , although done in solution.
Threads related to a possible reaction scheme.
http://www.sciencemadness.org/talk/viewthread.php?tid=1226#p...
http://www.sciencemadness.org/talk/viewthread.php?tid=1226#p...
http://www.sciencemadness.org/talk/viewthread.php?tid=1226#p...
Sodium nitrite ( NaNO2 ) melts at 271 ºC decomposing above 320 ºC.
If a lewis acid catalyst needs to be applied , anhydrous Aluminum Chloride
melts at 192 ~ 193 ºC but actually begins to sublime at 180. It is soluble
in organic chlorides.
.
[Edited on 15-10-2012 by franklyn]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | The possibility of substituting the chlorine with nitro groups . . . |
Or azide groups?
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ hissingnoise
I had not considered that and overlooked it. For a ' green ' primary it's worth the try.
Sodium Azide is very soluble in water 40g/100 ml at 20 °C
or liquid Ammonia , much less soluble in Acetone , DMF, DMSO ~1g/100ml at 20 °C ,
slightly in Alcohols < 1g/100ml at 20 °C
insoluble in ether.
NaN3 is reactive in Chlorinated solvents and are to be avoided
http://cen.acs.org/articles/88/i24/Sodium-Azide.html
Rate constants for displacement reactions by the Azide ion in DMSO
are up to about 10,000 times greater than for the same reaction in
protic solvents, such as Methanol. This merits consideration for use
in a Finkelstein halogen replacement reaction instead of Acetone.
DMSO's ability to bring molecules into the bloodstream transdermally
requires cautious use particularly carrying an Azide solute.
Solubility of Alkali & Alkaline Pseudohalides
www.nist.gov/data/PDFfiles/jpcrd643.pdf
Dimethyl Sulfoxide ( DMSO ) Handbook
www.gaylordchemical.com/uploads/images/pdfs/literature/105B.pdf
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Some tinker toy creations of unknown Nitrocarbon target molecules
which exhibit zero oxygen balance.
This illustrates the problem of choosing a destination , you then have
to figure how to get there. The Benzene variant below left , having two
sets of adjacent Nitroso groups , these would additionally condense
into two Furoxan groups , enhancing density. The Napthalene variant
below right has no adjacent Nitroso groups which can reform into
Furoxan as as this would incur steric hindrance between adjacent
Trinitromethane groupings.
P.S.
They look as if they will be rather toxic too.
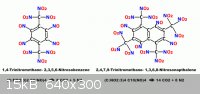
Some related compounds
www.sciencemadness.org/talk/viewthread.php?tid=2969#pid93082
www.sciencemadness.org/talk/viewthread.php?tid=11195&pag...
www.sciencemadness.org/talk/viewthread.php?tid=1226#pid17906...
www.sciencemadness.org/talk/viewthread.php?tid=1226#pid18257...
.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by franklyn  | | The Benzene variant below left , having two sets of adjacent Nitroso groups , these would additionally condense into two Furoxan groups , enhancing
density. |
Probably not worth even bringing up, but I have some doubts that two furoxan moieties can exist on directly opposite sides of a benzene ring.
Remember, the furoxan group becomes part of the aryl resonance, two furoxan groups in these positions might destabilize the resonance. Try
drawing a resonance state diagram to see what I mean.
The chemistry of nitrosyl groups are very different than the chemistry of furoxan groups, even though furoxan groups can seem to tautomerize
back and forth in some ways. Nitrosyl groups are normally easily oxidized to nitro groups, whereas furoxan groups are nearly impossible to oxidize.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
AndersHoveland
I guess you mean here ( green arrows )
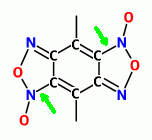
I have doubts too , why I left it with two nitroso showing
.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
hexanitro-hexaradialene revisited
Okay, I had this idea for hexanitro-hexaradialene as well and thanks to Dany, have been redirected here.
I think I have a suggestion for a synthetic methodology that if it could be shown to work, would be pretty neat:
My thoughts revolve around using quinone chemistry actually, mostly because I have experience in it and also because the reagents are simple and
cheap.
Firstly there's this little patent about chloranil:
https://www.google.com/patents/US5334735?dq=chloranil+hydrog...
I've performed this reaction several years ago and can confirm it works beautifully. It works so well because HCl can add to the quinone accompanied
by a reduction which drives it steeply 'downhill'. So for example, hydroquinone is oxidised to quinone by hot peroxide and then HCl adds to the ring
position and simultaneously reduces the quinone to hydroquinone resulting in the monochlorohydroquinone and then this continous on and on until the
quinone is saturated by chlorine, ultimately forming chloranil. This all happens in one pot until bam, a mass of choranil crystallises out... a rather
impressive synthesis considering it's simplicity.
Anyhow, I've also reacted the subsequently generated chloranil with sodium methoxide in methanol to form a mixture of tetramethoxybenzoquinone and
KCl, as well as the hemiacetal derivative thereof.
So..... this got me thinking, perhaps one could use nitromethane to attack the quinone and exhaustivly add to it.
Now the only question is what to use as oxidant... here we could take advantage of the following papers that show that hydroquinone can be oxidised by
oxygen rather efficiently using catalytic amounts of NO2 that is easy enough to introduce.
J. Org. Chem. 59, 2529-2536 (1994),
Tet. Lett. 35(9) 1335-1338 (1994)
Here they used DCM but I beleive nitromethane could work just as well perhaps. The nice thing about using NO2 as catalyst is that it would create
acidic conditions which can catalyse the isomerisation of the nitromethane into the reactive ene form.
And yes... I beleive this could be a one pot synthesis. Just like the exhaustive addition of HCl in the pressence of an oxidant. In this case, oxygen
or indeed even air is the safest bet as much more oxidation is required to evolve the final product compared to the chloranil case, but I see no
reason why this would not be at least plausable.
In another thread, I was trying to find out if anyone would be intrested to collaborate on this since I do not have the means to safely or legally try
it out.
[Edited on 22-4-2014 by deltaH]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Just as a side note your hexanitroradialene may add 6 extra nitromethane molecules to get dodeca-nitromethyl-cyclohexane (C6(CH2NO2)12); but also
polymeric materials....by reaction of -CH2NO2 group instead of CH3-NO2....
Aceton when added to one equivalent of nitromethane form:
(CH3)2C=O + CH3-NO2 --> (CH3)2C(OH)-CH2-NO2 --> (CH3)2C=CH-NO2 + H2O
But with two equivalents:
(CH3)2C=CH-NO2 + CH3-NO2 --> (CH3-)2C(-CH2-NO2)2
[Edited on 22-4-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Interesting thought, thanks PHILOU, I think it is highly likely that what you suggested would also occur with an exhaustive
nitromethylation of quinone under oxidising conditions.
Also, in hindsight, the autooxidation which quinones undergo with oxygen may stop whence the the quinone moiety is lost and so one might also not
oxidise the side nitromethyl groups. Combining these two thoughts would mean making this structure:
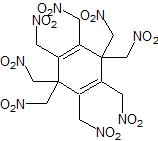
I'm disappointed with it now 
It looks like some kind of 'nitro-mite', probably low density, 1.5'ish, with a poor OB to boot  
I hate spiders   
[Edited on 22-4-2014 by deltaH]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by deltaH  | Interesting thought, thanks PHILOU, I think it is highly likely that what you suggested would also occur with an exhaustive
nitromethylation of quinone under oxidising conditions.
Also, in hindsight, the autooxidation which quinones undergo with oxygen may stop whence the the quinone moiety is lost and so one might also not
oxidise the side nitromethyl groups. Combining these two thoughts would mean making this structure:
I'm disappointed with it now 
It looks like some kind of 'nitro-mite', probably low density, 1.5'ish, with a poor OB to boot  
I hate spiders   
[Edited on 22-4-2014 by deltaH] |
OB can be enhanced by reaction with a nitrite salt in acetic acid or by reaction with a nitrite ester...that way each nitromethyl group becomes a
nitroso-nitromethyl one...
R-CH2-NO2 + R'-O-N=O --> O=N-CH(R)-NO2 + R'-OH
R-CH2-NO2 + HO-N=O --> O=N-CH(R)-NO2 + H2O
O=N-CH(R)-NO2 <==> HO-N=C(R)-NO2 <==> O=N-C(R)=N(O)-OH
So
C14H16(NO2)8 (or C14H16N8O16) becomes after per-nitrosation C14H8(NO2)8(NO)8 (or C14H8N16O24) 
Due to the acidity of such nitroso-nitromethyl groups...one might assume that it may form interesting amine salts (NH3OH, N2H5, NH4, ...) and
primaries metal salts (Li, Na, K, Hg, Ag, Pb, ...)
[Edited on 9-5-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks PHILOU, this is a clever trick you point out, I should remember it when thinking up nitromethane derivatives!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Reacting Radialene with Dinitrogen Tetroxide will get you 1,2,3,4,5,6-nitromethyl-1,2,3,4,5,6-nitrocyclohexane
[CH2(NO2)]6C6(NO2)6 => 6 CO2 + 6 CO + 6 H2O + 6 N2
Reacting Dodecanitroradialene with Dinitrogen Tetroxide will get you 1,2,3,4,5,6-trinitromethyl-1,2,3,4,5,6-nitrocyclohexane
Blending with Dicyanoacetylene then inducing that to polymerize will lock the nitrocarbon molecules within the polymer matrix.
[C(NO2)3]6C6(NO2)6 + 3 NΞC-CΞC-CΞN => 24 CO2 + 15 N2
The resulting material may resemble the rubber of truck tires
Dicyanoacetylene NΞC-CΞC-CΞN , http://www.chemspider.com/Chemical-Structure.13449.html
http://www.sciencemadness.org/talk/viewthread.php?tid=21743#...
.
|
|
|