moonlight_023
Harmless

Posts: 1
Registered: 29-7-2011
Location: France
Member Is Offline
Mood: No Mood
|
|
difference between Ph-Li and Ph-Mg-Br
Hello,
I read many organic synthesis but I have no theorical background.
In many synthesis, Phenyl Magnesium Bromide or Phenyl Lithium is used to react on some organo compound :
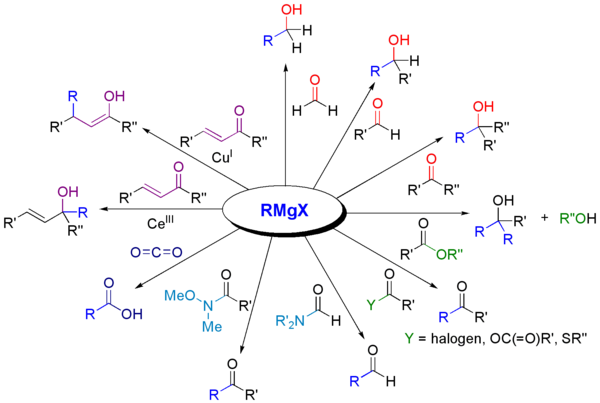

(the images come from wikipedia/grignard)
But most of time, it is not detailed if Ph-Mg-Br can be replaced by Ph-Li or not.
What is the main difference between PhMgX and PhLi (and in general between R-Mg-X and R-Li) ? Why in some reactions the one cannot replace the other ?
I guess it is due to a difference of electronegativity of the carbon, or to size of the leave group MgX/Li, or also to the spatial configuration of
the orgametallic with its solvent ?
Thank you !
R.
[Edited on 30-7-2011 by moonlight_023]
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
your question is pretty broad to give an answer to.
phenyl lithium will react faster but it also reacts with the solvents ether and thf grignards are easier to handle in terms of pyrophoricity.
and you can even do wet grignards under sonication.
it's a very broad subject.
Give me librium or give me meth!
Patrick Henry....
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Its worth reading a theoretical book once in a while, even if to just answer questions like this. However, on this occasion I'll oblige...
The difference in reactivity stems mostly from the difference in electronegativity between the metal and carbon; Lithium, the more electropositive
element, forms a more ionic bond than Magnesium, which itself shows appreciable ionic character. Other metals such as Copper and Cadmium form
organometallic bonds that are much more covalent in chatacter.
The more ionic the bond is in character, the "harder" the alkyl/aryl etc. fragment is (see Hard/Soft Acid/Base theory). This affects nucleophilic
preference (e.g. 1,2 vs 1,4-addition) and also the basicity of the reagent. n/s/t-BuLi is commonly used in organic chemistry as a strong base, whereas
grignard reagents are primarily used to do addition. The greater nucleophilicity of Organolithium reagents means that they (and they only) can be
added to carboxylic acids (given the correct reaction conditions; I suspect -78*C is standard) to afford ketones.
Solvation of the reagent is also a large issue when dealing with its reactivity. MeLi and tBuLi (as specific examples) are tetrameric in hexanes -
adding a coordinating solvent such as ether or THF (not for the latter as it metallates at the 2-position and subsequently fragments to ethylene and
the lithium enolate of acetaldehyde) produces a species that is dimeric if I remember correctly, and TMEDA (thats tetramethylethylenediamine) or HMPA
(hexamethylphosphoramide) can be added to produce largely monomeric species. The degree of oligomerisation affects the reactivity of the reagent.
Adding 1,4-dioxane as a solvent shifts the Schlenk equilibrium of Grignard reagents in order to furnish the dialkylmagnesium reagent, via
precipitation of the magnesium halide.
|
|
|
Bitburger
Harmless

Posts: 41
Registered: 23-7-2011
Location: Belgium
Member Is Offline
Mood: Experimental
|
|
I wonder why Knochel reactions use lithium chloride?
Good to be wrong
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Knochel type reactions mainly use iPrMgCl.LiCl. Plain iPrMgCl is dimeric in THF, whereas addition of LiCl breaks up the oligomer and also leads to a
slight increase of the negative charge on carbon. Hence the higher reactivity.
Anyway, the question in general is not so easy to answer. There are very important factors to consider.
1) Temperature. At low temperature organolithiums can be more reactive than at room temperature because of entropic effects.
2) Solvent and additives. Pretty much determine which species is actually present, from hexamer to dimer to monomer, greatly affecting reactivity.
3) Substrate. Most important effect is ortholithiation (although this effect is also noticeable with organomagnesiums) where a moiety that can
coordinate Li can shift the place of attack.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Bitburger
Harmless

Posts: 41
Registered: 23-7-2011
Location: Belgium
Member Is Offline
Mood: Experimental
|
|
Thank you very much. Really exciting and unique chemistry!
I fully understand, except the 3th point. I know that benzylmagnesium bromide + formaldehyde gives the corresponding ortho-alcohol of methyl benzene,
but I was always wondering why. Why is that ortho-hydrogen shifting in the case of formaldehyde, acetaldehyde and so on?
[Edited on 31-7-2011 by Bitburger]
Good to be wrong
|
|
|