| Pages:
1
2
3 |
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Strategies in Designing Ideal Explosives
When designing explosive molecules there are several strategies that are used to maximize explosive performance and physical stability. This topic is
for discussion about such strategies.
Incorporating Nitrogen
Nearly all explosives of any functional importance incorporate nitrogen into their molecular structures. Nitrogen atoms primarily serve as a way to
hold oxygen atoms to the molecule, so that the formation of carbon-oxygen or hydrogen-oxygen bonds will release more energy than the oxygen-nitrogen
bonds that need to be broken. The incorporation of nitrogen into a molecule can also compensate for lack of usable oxygen, as the formation of
diatomic nitrogen (N2) releases energy. Generally, explosives which contain more bonds between nitrogen atoms are much more powerful than those that
contain fewer bonds between nitrogen atoms. This may seem somewhat paradoxical because it is a common conception that nitrogen-nitrogen bonds are very
strong. More nitrogen-nitrogen bonds mean fewer other atoms are bonded to nitrogen. A single carbon-nitrogen bond is nearly twice as strong as a
single bond between two nitrogen atoms, while a carbon-nitrogen double bond is nearly 50% stronger than a similar nitrogen-nitrogen double bond. It is
only the triple bond between two nitrogen atoms that is stronger (only slightly) than a carbon-nitrogen triple bond.
Nitrogen-Hydrogen bonds
Amino groups can often act as electron donating groups, making explosives less sensitive. The hydrogen bonding in amino groups also greatly increases
intermolecular attraction, leading to higher densities.
Density
Just because a molecule is bigger and has more nitro groups does not necessarily mean it will be more powerful than a smaller molecule with fewer
nitro groups. Smaller molecules can often pack together to result in higher densities than larger molecules. For example, DADNE, which has the formula
C2N4H4O4, has a higher density and thus detonation velocity than RDX, C3H6N6O6. Caged molecules, such as TEX and HNIW, also tend to have higher
densities.
Molecular Strain
Another strategy that has been employed is designing molecules with strained bonds. Octonitrocubane is an extreme example of this. Strained bonds
require less energy to break. There has been some experimentation with triangular rings, but such compounds are generally much less stable. Cyclic
square rings are ideal. Pentagonal rings also have a lesser degree of strained bonds. Hexagonal rings, however, do not contain strained bonds, and are
in fact very stable.
The best potential molecules combine several different strategies listed above together. When designing molecules, it is best not to take any of the
above strategies to an extreme, but rather to incorporate elements from most of the strategies, to have a “well-rounded” design.
[Edited on 16-7-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Fusionfire
Hazard to Others
  
Posts: 219
Registered: 8-7-2011
Member Is Offline
Mood: No Mood
|
|
Oooh, many other comments, my friend 
1) In general, references/citations for your statements would be helpful for us non-chemists here 
2) Oxygen balance.
http://en.wikipedia.org/wiki/Oxygen_balance
TNT has an OB of -74% while AN has an OB of +20%
An OB deficit means that the explosive's potential is not being used to the fullest, IOW fuel is being wasted. An ideal explosive from an energetics
point-of-view would be one where either the molecule has an OB of zero, or it is mixed into a composite such that the OB is zero.
3) | Quote: | Nitrogen-Hydrogen bonds
Amino groups can often act as electron donating groups, making explosives less sensitive. The hydrogen bonding in amino groups also greatly increases
intermolecular attraction, leading to higher densities.
|
Not quite sure how electron donating groups de-sensitise explosives. Please elucidate.
4) Mean reactant diffusion distance. In solid molecular explosives, the diffusion distances are of molecular lengths. In thermites, it is of the order
of particle sizes. This has a direct implication on the reaction rate of the explosive.
5) Activation energy. Thermites have a high activation energy (both to overcome the passivation layer and to free the oxygen from the less
electropositive cation), hence their reaction kinetics are not ideal for use as explosives.
6) Enthalpy of the reaction. Higher heats evolved -> higher pressures evolved.
7) Moles of gas evolved per moles of reactant. More gas -> more destructive explosive.
Quote: Originally posted by AndersHoveland  | When designing explosive molecules there are several strategies that are used to maximize explosive performance and physical stability. This topic is
for discussion about such strategies.
Incorporating Nitrogen
Nearly all explosives of any functional importance incorporate nitrogen into their molecular structures. Nitrogen atoms primarily serve as a way to
hold oxygen atoms to the molecule, so that the formation of carbon-oxygen or hydrogen-oxygen bonds will release more energy than the oxygen-nitrogen
bonds that need to be broken. The incorporation of nitrogen into a molecule can also compensate for lack of usable oxygen, as the formation of
diatomic nitrogen (N2) releases energy. Generally, explosives which contain more bonds between nitrogen atoms are much more powerful than those that
contain fewer bonds between nitrogen atoms. This may seem somewhat paradoxical because it is a common conception that nitrogen-nitrogen bonds are very
strong. More nitrogen-nitrogen bonds mean fewer other atoms are bonded to nitrogen. A single carbon-nitrogen bond is nearly twice as strong as a
single bond between two nitrogen atoms, while a carbon-nitrogen double bond is nearly 50% stronger than a similar nitrogen-nitrogen double bond. It is
only the triple bond between two nitrogen atoms that is stronger (only slightly) than a carbon-nitrogen triple bond.
Nitrogen-Hydrogen bonds
Amino groups can often act as electron donating groups, making explosives less sensitive. The hydrogen bonding in amino groups also greatly increases
intermolecular attraction, leading to higher densities.
Density
Just because a molecule is bigger and has more nitro groups does not necessarily mean it will be more powerful than a smaller molecule with fewer
nitro groups. Smaller molecules can often pack together to result in higher densities than larger molecules. For example, DADNE, which has the formula
C2N4H4O4, has a higher density and thus detonation velocity than RDX, C3H6N6O6. Caged molecules, such as TEX and HNIW, also tend to have higher
densities.
Molecular Strain
Another strategy that has been employed is designing molecules with strained bonds. Octonitrocubane is an extreme example of this. Strained bonds
require less energy to break. There has been some experimentation with triangular rings, but such compounds are generally much less stable. Cyclic
square rings are ideal. Pentagonal rings also have a lesser degree of strained bonds. Hexagonal rings, however, do not contain strained bonds, and are
in fact very stable.
The best potential molecules combine several different strategies listed above together. When designing molecules, it is best not to take any of the
above strategies to an extreme, but rather to incorporate elements from most of the strategies, to have a “well-rounded” design.
[Edited on 16-7-2011 by AndersHoveland] |
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fusionfire  | Oooh, many other comments, my friend 
1) In general, references/citations for your statements would be helpful for us non-chemists here 
2) Oxygen balance.
http://en.wikipedia.org/wiki/Oxygen_balance
TNT has an OB of -74% while AN has an OB of +20%
An OB deficit means that the explosive's potential is not being used to the fullest, IOW fuel is being wasted. An ideal explosive from an energetics
point-of-view would be one where either the molecule has an OB of zero, or it is mixed into a composite such that the OB is zero.
|
Sigh. Tooooo many here are in love with numbers, and go
into sissy fits over detonation velocity & oxygen balance.
Both numbers have limited/specific practical meaning.
This from PATR-2700 (A free DL.)
     
No doubt the most misused term here do be
Explosive power a term which has a very
specific meaning.
djh
----
The WiZard is In - Is a
fan of none-dimensional
numbers. La deciBell - dB
(named after AG Bell's
daughter Deci.) is a
top of the list
|
|
|
Fusionfire
Hazard to Others
  
Posts: 219
Registered: 8-7-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by The WiZard is In  |
Sigh. Tooooo many here are in love with numbers, and go
into sissy fits over detonation velocity & oxygen balance.
Both numbers have limited/specific practical meaning.
|
Thank ye kindly for the reference, Wizard  . I read all of that. My comments
are: . I read all of that. My comments
are:
1) It can not be denied that fuel is being wasted when the OB of an explosive is substantially negative. The question is how to exploit this situation
without adversely affecting the explosive properties.
ETN (+5.3% OB) can probably be mixed with PETN (-10%) quite well without affecting the VoD of either.
I am not sure if AN (+20%) or NaNO3 (+47%) in a composite with TNT (-74%) to have an OB of zero would actually reduce the VoD or simply produce more
energy during afterburning and not detonation.
Therefore I agree with your reference when he says "OB correlates reasonably well with relative power, provided these comparisons are made for similar
expls."
BTW is there an error in Table 1 for TNT? It lists the OB there as -21.6% but just to the left the calculation showed it to be -74%
2) Figure 1 shows pretty good correlation between OB and explosive power.
As an engineer I understood the theoretical meaning of explosive power to mean the reaction heats divided by the reaction time. The reaction heat can
be calculated while I would estimate the reaction time to be the time taken for the VoD to traverse the sample of explosives, after making corrections
for DDT/SDT.
3) I'm not quite sure I agree with you that VoD has limited practical meaning. High VoD leads to faster hollow charge jets/EFPs, more brisance and
higher peak pressures. Hollow charges are the bread and butter of modern anti-armour weapons.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fusionfire  |
3) I'm not quite sure I agree with you that VoD has limited practical meaning. High VoD leads to faster hollow charge jets/EFPs, more brisance and
higher peak pressures. Hollow charges are the bread and butter of modern anti-armour weapons. |
Well ... I agree with myself! Given that US usage of explosives
and blasting agents is ca. 4 000 million pounds a year...
the small anount used in shaped charges meet the requirements of
"limited practical use." n'est-ce pas?
The most important factor for most is $$$$.
Explosives are tools - the one that does the best job for
the least price is the winner. Strange thing do however,
happen. Really expensive det cord is used to load holes to
blast rock! Lots and lots of small holes.
PLX is only used where its unique characteristic is useful,
the first (only) explosive used on the moon was carefully chosen,
like-wise the explosives used in lens for nuke warheads,
&c., &c....
Reminds me DuPont stopped making dynamite years ago,
it was tooo expensive to compete with ammonium nitrate
and aluminium slurry explosives.
|
|
|
Fusionfire
Hazard to Others
  
Posts: 219
Registered: 8-7-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by The WiZard is In  | Quote: Originally posted by Fusionfire  |
3) I'm not quite sure I agree with you that VoD has limited practical meaning. High VoD leads to faster hollow charge jets/EFPs, more brisance and
higher peak pressures. Hollow charges are the bread and butter of modern anti-armour weapons. |
Well ... I agree with myself! Given that US usage of explosives
and blasting agents is ca. 4 000 million pounds a year...
the small anount used in shaped charges meet the requirements of
"limited practical use." n'est-ce pas?
The most important factor for most is $$$$.
Explosives are tools - the one that does the best job for
the least price is the winner. Strange thing do however,
happen. Really expensive det cord is used to load holes to
blast rock! Lots and lots of small holes.
PLX is only used where its unique characteristic is useful,
the first (only) explosive used on the moon was carefully chosen,
like-wise the explosives used in lens for nuke warheads,
&c., &c....
Reminds me DuPont stopped making dynamite years ago,
it was tooo expensive to compete with ammonium nitrate
and aluminium slurry explosives.
|
Well, bringing out the AN rabbit from the hat for quarrying is kind of cheating  .
When designer explosives were mentioned in the OP I thought "high performance regardless of cost" (otherwise octanitrocubane, et al wouldn't have been
mentioned) .
When designer explosives were mentioned in the OP I thought "high performance regardless of cost" (otherwise octanitrocubane, et al wouldn't have been
mentioned)
AN is produced in mass quantities for both agricultural and mining industries. It is also really insensitive, meaning that production is less
complicated than more orthodox secondaries. This means it is safe to make and safe to (ab)use.
Chemically it is very simple too, and I'd hazard a guess as a non-chemical engineer that there is a strong correlation between complexity of the
organic molecule (say measured by a combination of functional groups + MW) and cost to produce.
And of course civilian markets tend to be much larger than military ones.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
But not trivial.
Pueblo Army Depot
High Explosives Assorted 400 000 000 pounds.
Report of Ulta-Hazardous Substances at Federal Installations
in Colorado
EPA 18 April 1972
PB-255253
Years back I was on the mailing list (snail-mail) for US Gov
surplus explosives. You would be amazed at the tonnage of
scrap C4 there was offered for sale. Yahooo! Never go around
to finding out who purchased it.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Take good notes there will be ....
a test. But first let me get atop my High horse.
Brisance like Sabot, Fresnel (lens), Mine ball
are French words they are not pronounced the way
a speaker of English would pronounce them if they were
English words. Don't look like a amateur look up its (their)
pronunciation.
Extra credit try isochronous and Oaxaca.
     
|
|
|
Fusionfire
Hazard to Others
  
Posts: 219
Registered: 8-7-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by The WiZard is In  | a test. But first let me get atop my High horse.
Brisance like Sabot, Fresnel (lens), Mine ball
are French words they are not pronounced the way
a speaker of English would pronounce them if they were
English words. Don't look like a amateur look up its (their)
pronunciation.
Extra credit try isochronous and Oaxaca.
|
Oooooh you are too kind, thank you again for another reference 
Delightful reading material. Will respond tomorrow once I have had a chance to read & digest it.
BTW what reference management system do you use? What is the going rate of a mirror of your entire reference collection?  
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fusionfire  |
BTW what reference management system do you use? What is the going rate of a mirror of your entire reference collection?  
|
Ref management system... mostly between my ears
backed up by searching my HD and Google/Deja for the stuff
I posted in the past without saving to my HD.Also eye-balling
my book shelves a plenty.
Mirror of your entire reference system?! A Vulcan Mind Meld perhaps.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Of course oxygen balance is important, but the concept can be very misleading. While all the hydrogens should ideally be oxidized to H2O, it is much
less important to oxidize the carbons all the way to CO2. Formation of carbon monoxide is sufficient. As can be seen below, the inclusion of six atoms
can lead to 2 molecules of carbon dioxide, or alternatively to 3 molecules of carbon monoxide, which will occupy a greater volume after decomposition.
(2)C + (4)O --> (2)CO2
(3)C + (3)O --> (3)CO
Despite this, explosives which result in CO2 as the decomposition product still tend to be slightly more powerful, but it also requires that more
usable oxygen be incorporated into the molecule, and this typically leads to greater sensitivity as more nitro/nitramine groups are required. The
trade-off is generally not worth it.
And there do exist several powerful tetrazole explosives that do not even contain oxygen. The carbon in these molecules does not really need to be
"burned off" because the formation of HCN as decomposition product releases nearly as much energy as formation of N2.
While C--H bonds can potentially generate much more energy on being oxidized than N--H bonds, they also require more oxygen. If the molecule is
oxygen-deficient it may be more practical to substitute out some of the carbon with nitrogen atoms instead.
Geminal Nitro Groups
Putting two nitro groups on the same carbon atom is another strategy that has been employed to improve oxygen balance. Although such compounds still
tend not to be very sensitive to initiation, there exists the problem of thermal stability, where there is slow decomposition in storage under warm
conditions, or rapid degredation if the explosive becomes subjected to the heat from flames outside the metal casing.
Geminal-dinitro refers to two nitro groups on the same carbon atom. Gem-dinitro groups show good potential for incorporation into the structures of
new explosive molecules and propellants. Molecules containing either mono- or di-nitro alkanes are generally much less sensitive than nitrate esters,
while still being quite energetic when detonated.
There are two primary reasons that nitro groups are not often incorporated into typical explosive molecules. The first is that, in many cases, it is
much more complicated to introduce more than one nitro group onto the same molecule. While aromatic rings are easily nitrated into corresponding di-
and tri-nitro compounds, most other molecules are much more difficult to nitrate to nitro compounds. Substitution reactions, in which a bromoalkane
reacts with nitrite ions, give satisfactory yields for single substitution, but the yields greatly decrease for di- and tri- nitro substitution. The
most energetic mono-nitro alkane is nitromethane, which has a significantly lower density relative to other common explosives. Simple nitrations with
mixed acids generally fail to produce nitro alkanes. This is because of the Meyer reaction, in which (R)2CH(NO2) groups disproportionate under acidic
conditions, oxidizing the carbon to leave either a ketone or carboxyl group. Molecules in which the carbon bonded to the nitro group is also bonded to
three other carbon atoms are not vulnerable to this type of disproportionation. An example of such a molecule would be (CH3)3C(NO2).
The second reason is that most, but certainly not all, molecules which contain the gem-dinitro group are not thermally stable, despite usually being
fairly insensitive to impact.
The examples of stable gem-nitro molecules seem to have one thing in common. In all cases, elimination of HNO2 and resultant formation of an
unsaturated C=C bond, is not possible. In other words, the molecules lack an (R)2CHC(NO2)2CH(R)2 segment, or if such a segment does exist, the
carbon-carbon bonds are under a high degree of strain.
Dinitropropanes that do not have a hydrogen atom on the same carbon as the dinitro group require a higher temperature for thermal decomposition than
those that have such a hydrogen. P. S. DeCarli, D.S. Ross, Robert Shaw, E. L. Lee, H. D.Stromberg. For example, the solid compound 2,2-dinitro propane
is thermally unstable when warmed. At 75degC, it partially decomposes, losing two thirds of its weight after two days. There are, however, several
conditions under which gem-dinitro compounds can be thermally stable. In constrast, dinitromethane shows little thermal instability at room
temperature, and the pure liquid shows no sign of decomposition after being stored for several months at 0degC. Dinitromethane is, however,
significantly less thermally stable than mono-nitro alkanes.
Adding a fluorine atom to the gem-dinitro group, with a structure –CF(NO2)2, greatly lends stability to the gem-ditro group. An example of this is
the energetic plasticizer bis(2-fluoro-2,2-dinitroethyl) formal (FEFO), which has excellent thermal stability. FEFO decomposes first at 150 ° C by
rearrangement of the nitro group in the loss of nitric oxide and nitrite. Nitrogen dioxide is also formed at 170 ° C.
Other examples of gem-dinitro compounds without any thermal stability problems include 1,1-diamino-2,2-dinitroethylene (DADNE) and
1,3,3-trinitoazetidine (TNAZ). In the first case, the amino groups act as electron donors to the nitro groups through the carbon-carbon double bond.
The molecule is effectively aromatic, which is indicated by the yellowish color of the pure compound. An extra electron is a gem-dinitro group,
whether as an anion such as in the salt potassium dinitromethanate (K+ O2NCH=NO2- ), or present in an aromatic compound, greatly adds stability to the
compound, both thermally and in terms of resistance to detonation. In the case of TNAZ, the high bond strain from the square ring configuration
creates a high energy barrier for a hydrogen atom to ionize off and leave a double carbon-carbon bond as the extra electron reduces one of the nitro
groups to a nitrite anion. In such instances, the -CH2—C(NO2)2— segment of the molecule, eliminate eliminates nitrous acid (HONO), leaving behind
–CH=C(NO2)—. Formation of an unsaturated bond is much more difficult when there is a high degree of bond strain.
[Edited on 17-7-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Some times it the simple thing that counts...
through WW I the explosive of choice for bombs/shells was
picric acid. PA was rapidly replaced by TNT, not because TNT
had higher velocity of detonation, brisance, power, higher/lower
oxygen balance .... TNT was/is used simple because it has a
much lower melting point then PA.
Granted PA lived on in Explosive D, however, with the collapse
of Ship to Ship Gunnery, Dunnite has very little if any current use.
djh
----
Finally, there is the case of explosives
scientist who fabricated an ash tray from
cast TNT and kept it on his office desk
for the use of visitors, only revealing its
nature after they had extinguished a
cigarette in it with no untoward results.
H.J. Yellop
Explsion Investigation
The Forensic Science Society, 1980.
[Edited on 17-7-2011 by The WiZard is In]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Of course there are other properties of explosive compounds that must be considered, besides just explosive performance and sensitivity.
Melt-castability, thermal stability, toxicity, and chemical reactivity are important properties.
The energetic plasticizer FEFO, for example, is an ideal explosive except for two serious problems; it is extremely toxic, and it will slowly corrode
virtually every type of container it could be placed in, including both glass and gold. It even seeps through and saturates teflon, leading to
structural weakening of the polymer.
Trinitroanaline, despite initially being a very insensitive explosive, can spontaneously detonate during storage from a spontaneous run-away chemical
degredation.
Picric Acid, while not too sensitive itself, can be dangerosuly sensitized by traces of metallic contaminants or by coming in cotact with brass/copper
metal.
Hygroscopicity and stability in air are also considerations; TACN becomes difficult to detonate after being exposed to moist air, while acetone
peroxide slowly sublimes away if left in an open container.
quote by Fusionfire: "Not quite sure how electron donating groups de-sensitise explosives."
Electron donating groups can introduce an extra electron into nitro groups, which greatly further stabilizes the nitro group.
The structure of DADNE is often shown as (NH2)2C=C(NO2)2,
but it would be more descriptive to show it as [+]NH2=C(NH2)--C(NO2)=NO2[-]. A similar structure could be shown for triamino,trinitro,benzene (TATNB)
which is extremely insensitive. Consider that a perchlorate anion, ClO4[-], is much more stable than Cl2O7. The stability is a result of an extra
electron.
Hydrogen bonding also, by increasing intermolecular attraction, makes explosive substances more difficult to ignite. (To state the obvious, note that
hydrogen atoms bonded to carbon do not engage in "hydrogen bonding")
Electron donation also typically results in more polar molecules, meaning higher density.
[Edited on 17-7-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Fusionfire
Hazard to Others
  
Posts: 219
Registered: 8-7-2011
Member Is Offline
Mood: No Mood
|
|
Some parameters can be fiddled around with, with composite explosives. E.g. kieselguhr to desensitise NG hence dynamite, likewise plasticisers for RDX
to improve formability hence PE4.
Toxicity and chemical reactivity are probably harder to deal with.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Arrived late to this lively discussion
Just my 2 cents, Fusionfire pointedly observes that
" 1) It can not be denied that fuel is being wasted when the OB of an explosive is substantially negative."
this is equivalent to mixing a high grade explosive with inert binder.
Given that enthalpies of formation of the explosives in common use
all cluster around zero, it follows that whatever energy that can be
mustered must necessarily derive from autocombustion.
Exotic enhancements such as strain in caged formations and extreme
endothermicity for now remain relegated to experimental investigation.
Density is the one property which directly affects detonation pressure
and can be selected from available prospective candidate compounds.
Velocity is the best available rule of thumb in ranking explosives, most
closely gauging the performance exhibited by the various other testing
regimes.
.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I am just of the opinion that, while oxygen balance is certainly an important consideration, this particular property is being overemphasized by both
professional researchers and by many in this forum. Ideal oxygen balance should not be pursued at the complete expense of neglecting all other
strategies. This misconceptions likely stems from the fact that most all common explosives in use have relied almost exclusively on this single
property. But to design better molecules for the future, the horizons will have to be expanded. It is much more difficult to synthesize molecules
which incorporate the other strategies, however.
[Edited on 17-7-2011 by AndersHoveland]
I'm not saying let's go kill all the stupid people...I'm just saying lets remove all the warning labels and let the problem sort itself out.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by AndersHoveland  | | Picric Acid, while not too sensitive itself, can be dangerosuly sensitized by traces of metallic contaminants or by coming in cotact with brass/copper
metal. |
Is that right? How big are the traces? Tell me/us all about it please. However, let us first be clear on the rules for substantiation ...no
bullshit references or propaganda sources that are incorrect are acceptable. Only good confirmed reliable accurate information is allowed.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by The WiZard is In  | through WW I the explosive of choice for bombs/shells was
picric acid. PA was rapidly replaced by TNT, not because TNT
had higher velocity of detonation, brisance, power, higher/lower
oxygen balance .... TNT was/is used simple because it has a
much lower melting point then PA. |
The main reason for the demise of PA in munitions was its relatively high sensitiveness.
In WWI, the British found that the German TNT-filled shells could penetrate a ship's armour, exploding within, causing great damage, whilst their
PA-filled munitions exploded on contact and inflicted little damage . . .
A lesson learned?
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  |
In WWI, the British found that the German TNT-filled shells could penetrate a ship's armour,
exploding within, causing great damage, whilst their PA-filled munitions exploded on contact
and inflicted little damage . . . |
That's very deceptive or else you draw the wrong conclusion.
The only self initiated munitions used back then were panclasite
filled aircraft bombs.
Ever hear of an explosive train ?
Shells explode when their fuzes set off the filler.
The reasoning of the British was to breach the
hull to sink the vessel. The reasoning of the
Germans was to set the vessel ablaze.
Dealers choice.
Anyway after Jutland both saw little action.
____________________________________
Please lets not turn this into another PA " sensitivity " thread
That on going dispute can be argued here _
http://www.sciencemadness.org/talk/viewthread.php?tid=13187
.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Sandia National Labs did a great deal of work on what was NOT 'Ideal" when examining defense systems toward both terror and "standardized warfare".
In fact, some of the most common definitions utilized today & examinations of utilitarian energetics originate from Sandia.
There is a great deal of information via SNL.
[Edited on 18-7-2011 by quicksilver]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
PA is energetic materials 101. You either know some good information about it or
else you only think you know what you have read, a lot of which information isn't really knowledge but only regurgitated error masquerading as
knowledge. And because our friend AH has a tendency might I say to reach a bit far beyond literature which may or may not even be understood, we get a
rich mixture there of what is known and what is not known absent any clear distinction. So no, it is not my purpose to make debate anew about what
there really isn't any debate, but to underscore the overreaching theory that can occur absent fact checking.
There is a difference between getting a lot of things right and being expert enough to teach and I particularly dislike the knowledgeable professorial
tone from those who know a fair amount about some things they are talking while obviously to everyone but themselves don't know shit about the rest,
but just keep talking like they don't know when that boundary has been crossed between what they actually do know and what they actually only surmise
to be so... but which may not be so at all.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Oxygen balance vs Q

djh
---
It is interesting to note that for some
primary explosives, like lead azide, lead
styphnate, and mercury fulminate--and
secondary explosives, like PETN and
TNT--that the secondary explosives melt
at a temperature below the point at
which they normally explode, whereas
the primary explosives explode below
the temperature at which they melt,
This may help to explain the marked
difference in sensitivity between the
two types of explosive. However,
melting point alone cannot explain the
differences in sensitivity of pure
secondary explosives; for example, ....
AMCP 706-180
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Attachment: Relationship between Brisance of Explosives and their Molecular Atomic Structures.pdf (890kB)
This file has been downloaded 1107 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Regarding oxygen balance it is very succinctly stated by AndersHoveland
" most all common explosives in use have relied almost exclusively on this single property.
But to design better molecules for the future, the horizons will have to be expanded. It is
much more difficult to synthesize molecules which incorporate the other strategies, however."
An interesting question that remains to be resolved is whether it is better
on the whole to pursue zero oxygen balance or forego this in favor of
producing a somewhat greater number of molecular detonation products.
Here is an example :
Tetracyanoethylene is a commercially available reagent.
- Heat of formation = + 148 Kcal/ mol, average of three values
Given it's electron deficit character as observed here _
www.nature.com/nature/journal/v199/n4892/abs/199483a0.html
it should readily undergo cycloaddition with another unsaturated
olefin such as Tetranitroethylene to form TetranitroTetracyanoCyclobutane.
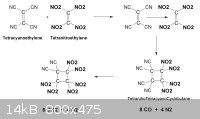
Detonation of that yields 12 gas mols per molecule.
If two of the nitriles are replaced ( somehow ) with nitro groups , that
compound is oxygen balanced , but detonation yields just 10 gas mols
however the heat of explosion is twice greater. A rough comparison
- 94 Kcal/ mol for CO2 , 6 CO2 , is - 564
- 26.4 Kcal/ mol for CO , 8 CO , is - 211
Tetracyanoethylene
www.wolframalpha.com/entities/chemicals/tetracyanoethylene/6...
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1263...
www.chemicalbook.com/ProductChemicalPropertiesCB9854093_EN.h...
http://webbook.nist.gov/cgi/cbook.cgi?Name=TCNE&Units=SI
www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0877
www.dtic.mil/docs/citations/ADA197044
Tetranitroethylene is very difficult to make and even more unstable than
Tetracyanoethylene , deteriorating away in a matter of days.
I can find no data as to Heat of formation , I estimate it at + 16 Kcal/ mol
A, Villiers, Bull. Soc Chim., 43, 322-324
T. S. Griffin and K. Baum, J. Org. Chem., 45, 2880 (1980)
K. Baun and D. Tzeng, J. Org. Chem., 50, 2736 (1985)
Tetranitroethylene was generated from hexanitroethane in the presence
of anthracene in refluxing benzene and in the presence of cyclopentadiene
in methylene chloride at -1O ºC.
Baum and Tzeng synthesized Tetranitroethylene in 50% yield via the flash vacuum
pyrolysis of hexanitroethane. The optimum conditions for the synthesis involve
passing hexanitroethane vapor, at 1 mm Hg, through a pyrolysis tube heated to
240-270 °C and condensing the product, a greenish yellow solid, in a cold trap.
Nitrocarbons - Arnold T. Nielsen , ed.
www.amazon.com/Nitrocarbons-Organic-Chemistry-Arnold-Nielsen...
Tetranitroethylene is discussed in Chapter 3 , page 87 preparation , page 89 properties
Heats of formation
http://garfield.chem.elte.hu/Burcat/burcat_old/hf.doc
.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Misspellings are pretty toxic to credibility - and rightly so . . .
|
|
|
| Pages:
1
2
3 |
|