| Pages:
1
2
3
..
5 |
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
BIODIESEL - cheapen your gasoline
I admit that's a bit of a catch phrase, but researchers all over the world are working on economical ways of doing this. Basically, it proabably would
be economical if it was done on a large scale, with genetically engineered plants that'd produce vast amounts of oil.
In fact, biodiesel can be made easily at home, at good quality and good price, providing you can buy ethanol/methanol, vegetable oils and a base or
acid such as Na/KOH or HCl in bulk.
The below information is from a friend who researched this for his diploma (with his permission), so I condensed nearly 50 pages to this. Enjoy 
Introduction:
Vegetable oils are glycerol esters, such as mono, di and triglycerides. The acids esterifying the glycerol are long chain carboxylic acids such as
oleic, palmitic, stearic acids (and many more, where variations are in the length of the chain (between 12 and 20 carbons), and the number (if any) of
unsaturated bonds.
In principle, the greater the number of double bonds, the lower is the melting point & viscosity of the oil. Same goes for chain lengths.
The ester bonds of the glycerides can be broken, to yield free glycerol and free carboxylic acids. The carboxylic acids can be esterified again, with
a an alcohol such as ethanol/methanol. The Metyl/ethyl esters of the fatty acids is what is referred to as Biodiesel !
The reaction equations are
Tri-glyeride (= Oil) + Methanol ---> Fatty Acid Methyl Ester (= Biodiesel) + Glycerol
MeOH + Triglyceride --> Biodiesel + Diglyceride
MeOH + Diglyceride --> Biodiesel + Monoglyceride
MeOH + Monoglyceride--> Biodiesel + Glycerine
Why is Biodiesel preferred to the conventional one?
The raw materials contain virtually no sulphur compounds, so no SO2 is produced. SO2 emissions from transport, especially from conventional diesel
fuel, are responsible for a large fraction of acid rain caused by SO2 (indeed in some countries this is an issue  ) )
Biodiesel contains about 10 wt-% of oxygen. This, combined with a high degree of unsaturated carbon double bonds, leads to a more complete combustion
than conventional diesel fuel. CO, hydrocarbon and particle emissions are hence greatly reduced. This is especially true for less sophisticated diesel
engines that have less favourable combustion conditions.
What I found VERY interesting is this: Biodiesel is completely compatible with modern diesel engines. In fact, the cetane number is even higher
than with conventional diesel resulting in a smoother running of the engine.
The reaction, and reaction setup
Choice of Alcohol:
Methanol and Ethanol are equally suitable for the usage for biodiesel, but the choice has been generally methanol due to its lower cost. Butanol is
another option.
Choice of Oil:
Here, Sainsbury's Rapeseed oil was chosen. Check it, it's definitely cheaper than petrol!
Choice of catalyst:
Three types of catalyst can be distinguished: alkali, acid and enzyme. Alkali-catalysed reactions are much faster (about 200 times) than
acid-catalysed. However, acid-catalysed reactions can deal much better with low quality raw materials, especially oils containing significant levels
of free fatty acids or moisture. Industrially acid catalysis is used (at high pressures etc) while here, KOH was the catalyst of choice due to fast
reaction rates.
Lurgi Process
This alkali based process uses high quality refined vegetable oil and operates at ambient pressure and about 60 to 70 °C.
The purpose of the reflux condenser was to prevent evaporating methanol escape from the reactor, especially at higher temperatures and/or longer
reaction times.
The setup is shown below:

The oil comprised betw. 80 and 90% of the reaction, depending on conditions (see below).
Molar ratios of oil vs methanol:
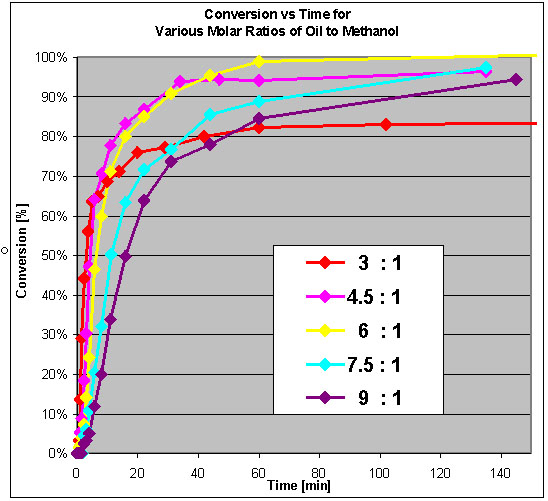
It can be seen that a decent excess of alcohol vs oil is desired, to achieve fast conversion. I have to find out whether the ratio 6:1 refers to
methanol vs triglyceride or methanol vs fatty acid.
Effect of overall catalyst/KOH concentration
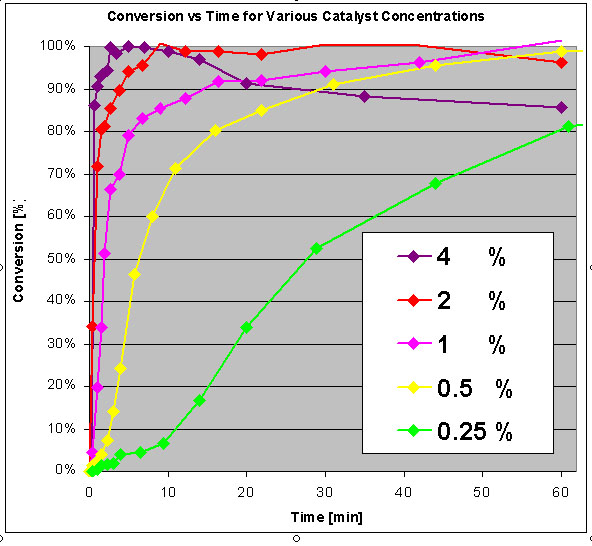
Here a final concentration of 2% (weight/weight presumably, or 0.5% per mass oil) is most desirable, at lower concentrations the conversion rate will
be slow, at higher concentrations saponification will occur (which means the methylester/biodiesel is hydrolysed itself)
Effect of temperature
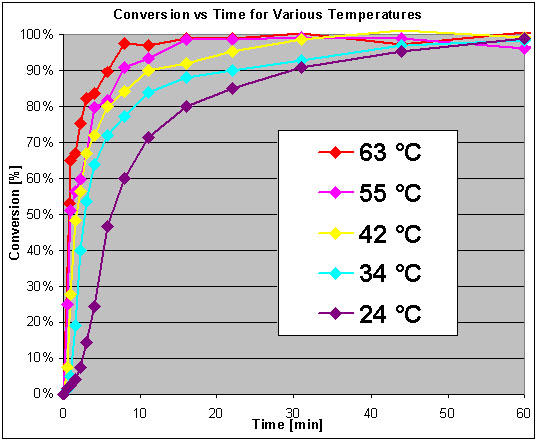
Here it's quite clear the higher the temp. the faster the reaction rate.
Conclusion
If you do the biodiesel production (fatty acid methyl/ethylester) yourself, aim for a 6:1 molar ratio alcohol vs vegetable oil, with 2% KOH (which is
added to the reaction mix as a saturated KOH solution, to minimise H2O), and elevated temperatures.
The reaction then should be complete after 20 minutes at most.
Purification of the fatty acid methylesters (biodiesel):
This is quite simple. The heavy dense glycerol settles to the bottom of the flask, while the methyl/ethyl esters remain above

The remaining biodiesel can be neutralised with weak acids, until pH 7 is achieved (the unneutralised esters have a pH of 8.) Alternatively, and
possibly preferably, the biodiesel is washed numerous times with water, and the biodiesel is decanted after the emulsion has separated into two
layers.
Use of biodiesel in Combustion Engines
In this particular quest, the biodiesel was used with model combustion engines. An improved performance was shown (!!) despite the original combustion
engine using an optimised oil of
25 % Castor oil
40 % Paraffin
33 % (Di-ethyl) ether
2 % Isopropyl nitrate
Anyway, I realise this is only a preliminary study (and so does the original author).
I hope nonetheless that someone tries that for himself, let's see whether you can get the old lawnmower running with biodiesel!!
[Edited on 17-3-2004 by chemoleo]
[Edited on 14-8-2011 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
There are many people who run their diesel engines with plain vegetable oil without refining thus. (or at least a mix of 80% vegetable and 20% diesel)
There are some minor modifications necessary - a preheater for the oil and thicker fuellines - bur overall it is easy and can save a lot of money.
There is a switch to change between diesel and vegetable oil - this makes starting in the wintertime easier. Most diesels have no problem at all
burning the stuff.
I will try to find the (german) webpage again.....
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Yah, I remember, this is what they used to do a while back, when ordinary fuel was scarce. I remember that the tractors that use vegi oil itself had a
different sound/smell.
I guess the advantage of using proper biodiesel (methylated fatty acids) is that the methylesters have
a) lower viscosity
b) better flammability
c) and better ignitability at lower temp.
d) and still cheap
e) Plus, the cetane number being higher.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
How does the price compare to ordinary diesel when you take taxation into account? In the UK (I'm not sure about other countries), anything used
to fuel a car is subject to fuel duty.
See:
http://ww2.green-trust.org:8383/2000/biofuel/Biofuel_UK_Tax....
http://news.bbc.co.uk/1/hi/wales/2310095.stm
1f `/0u (4|\\| |234d 7|-|15, `/0u |234||`/ |\\|33d 70 937 0u7 /\\/\\0|23.
|
|
|
Al Koholic
Hazard to Self
 
Posts: 98
Registered: 2-12-2002
Member Is Offline
Mood: Seeking ligand
|
|
I'm also fairly certain that the biodiesel fuel burns hotter than normal diesel. Yes you are eliminating oxidized S compounds, yes you have
better combustion completion, but you have increased emission of NOx.
Also, on a large scale, the process becomes quite problematic in terms of over all energy efficiency, especially when compared to petrol derived
diesel. The meer collection, processing, and reactions to produce a gallon of biodiesel usually make it prohibitavely energetically costly. I would
say the main problem being the initial harvesing and transportation of the feedstock because it would contain such an incredibly low energy density
when compared to crude.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
| Quote: |
yes you have better combustion completion, but you have increased emission of NOx. |
Why would this be? I thought, at higher temperatures, the NOx would decay?
Anyway, in some countries exhaust catalysers are used, which essentially eliminate the NOx problem (Ever noticed the pungent small coming from a non
catalysed exhaust?).
With regard to cost and efficiency of processing, of course it can't complete with fossil petrols yet. The point will come, however, when the oil
is used up, such renewable sources will become more feasible.
Don't forget, the petrol/oil industries had decades of research and experience, and zillions in investments, which make the production relatively
cheap. If we did the same to renewable sources, I am sure such renewable energies would become economically feasible in the near future...but this is
not how the dollar rolls, unfortunately...
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Al Koholic
Hazard to Self
 
Posts: 98
Registered: 2-12-2002
Member Is Offline
Mood: Seeking ligand
|
|
I can't find any specific mechanistic data right now on specifically how temperature affects the oxidation of atmospheric N2, but I beleive that
the higher the temperature, the more N2 will be oxidized in a given time to NO/NO2. Biodiesel burns hotter than normal diesel and hence has higher
NOx output.
In fact, B2, B20, and B100 are the common biodiesel fuels, the number after the B indicating the percentage of biodiesel in the fuel (which is
obviously mixed with petro diesel when not B100). The B2 fuel increases NOx emissions by a very small amount while the B20 and B100 increase NOx by
2 and 10% respectively when compared to standard petrol diesel. According to http://www.agriculture.state.ia.us/biodiesel.html , the timing of the engine cycle can help to reduce the NOx emissions somewhat but it is still
more than petro.
Catalysts would most definetly help and to be honest, I think advances here would help bring this issue under control in the future.
It is unfortunate that the biofuels are in the situation they are in IMHO. The expenses revealed by a thourough cost analysis sure set back their use
considerably when compared to crude products but the ultimate position they will occupy in the world economy could turn out to be very pivotal when
crude prices skyrocket. Investments in means of making the overall process more efficient and cost effective would surely pay off to some degree in
the future oil crisis.
I am an advocate of hybridizing technologies such as solar, geothermal, wind, etc with chemical technology as I see this turning, for example solar,
into a much more versitle power source. The ultimate feasibility of these options remains to be seen on a large scale but I am nearing the completion
of plans to do small scale tests with solar distillation and such...
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
At the moment, here in the UK it is only cheaper to use cooking oil than diesel because you don't pay duty on cooking oil. This is ilegal. OTOH
there are some people who use recycled oils and pay the duty. It still works out cheaper because they don't have to pay to have the used oil
disposed of.
As the world runs out of petroleum I think there will be a shift towards renewable fuels but I'm not sure which ones they will use (methanol and
hydrogen are the usual sugestions).
Here's an interesting point; the CO2 from biodiesel is more radioactive than that from traditional diesel.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Yeah C14 enriched oils  . .
Actually your points are good unionised...
Why bother with vegetal oil,low molecular weight alcohols, catalyst,heat, agitation, time to react and decantate, washings and acumulate
glycerol...when methanol, ethanol and aceton already are cheaper and choice fuels...
CH3-OH is a good choice
CH3-CH2-OH is a better choice
They burn too hot? Add some % water.
No NOx nor SOx troubles.
Easy available via conventional distillation.
For sure cheaper than your biodiesel because they are amongst the starting materials...you spare all the process costs.
From the above one can make...
CH2=O, CH3-CH=O and then in acidic conditions acetals like...
CH3-O-CH2-O-CH3, CH3-CH2-O-CH2-O-CH2-CH3, CH3-CH(-O-CH3)2, CH3-CH(-O-CH2-CH3)2
(Use of peroxydation inhibitors must be done to avoid peroxydes formations.)
But also...
HCO2H and CH3-CO2H
From what HCO2-CH3 (methyl formate), HCO2-CH2-CH3 (ethyl formate), CH3-CO2-CH3 (methyl acetate), CH3-CO2-CH2-CH3 (ethyl acetate)...Ahhh good smelling
ester fuel...(rhum aroma-and fruity smells) 
Final step might be CH3-CO-CH3 from acetic acid...although it is poor overal weight yield since for every association of two molecules of ethanoic
acid (acetic), you have to loose CO2 and H2O.On the other hand aceton can be obtained by fermentations too...
The later, aside with CH3-CH=O offers interesting crotonisation reactions in alakline or acidic media...
(CH3)2C=CH-CO-CH3, (CH3)2C=CH-CO-CH=C(CH3)2, CH3-CH=CH-CH=O, (CH3)2C=CH-CH=O, CH3-CH=CH-CO-CH3, CH3-CH=CH-CO-CH=CH-CH3, ...
are the potent resulting molecules.
Hydrogenation might then lead to corresponding saturated alcohols or unsaturated ones; methanol or ethanol will lead in acidic media to corresponding
acetals.
So starting from fermentation you can get a lot of other cheaper bio-fuels equivalent to benzine.
*****************************************
Hydrogen is another very good alternative fuel also quite cheap and easy to make.
*****************************************
The process you exposed is simply a reaction of transesterification.... methanolisation or ethanolisation thus....
What about recycling glycerol into triformic or triacetic ester?
Tributanoic ester is major ingredient of butter...so everybody can make glycerol and butyric acid at home...the later is a good fuel except it has a
stinky repulsive smell of rotten butter.
   
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
You could do any or all of those nice interesting reactions, or you could just run diesel engines on vegetable oil. I wonder which would be cheaper?
Methanol and such are only cheap because we haven't run out of oil yet.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Methanol is made from C=O and H2 or from wood distillation...if you don't know the origin of the name methanol, it comes fomr the old latin word
methus (spirit of wood).
Ethanol might be done by suggar fermentation or via ethene(oil dependant yes) hydratation with acids
So really no need of petrol oil for methanol or ethanol.
Even in the case we are runnig out of oil...methanisation of shit is a natural source of methane...
.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by PHILOU Zrealone
CH3-CH2-OH is a good choice
|
I instantly thought of Homer Simpson when I read this, remember the "one for you, one for me" -(homer fueling an ethanol fueled car)
http://www.dangerouslaboratories.org/
This site states that when the sodium hydroxide it mixed with the methanol that sodium methoxide is formed. I was under the impression that the
sodium hydroxide was a catalyst. All in all their procedure lookes like it works based on the two layes formed at the end despite getting the
reaction mechanism wrong.
[Edited on 3-6-2004 by rogue chemist]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Sure, ethanol is definitely a viable alternative to the biodiesel described above.
However... the big advantage of biodiesel is that it can be used STRAIGHT on existing diesel combustion engines, while methanol/ethanol can't.
That's why biodiesel gets so much attention. Plus, from rape plants (is that the word?), one not only gets glycerol and the fatty acid esters,
but ALSO lots and lots of biodegradable plant mass, from which MORE ethanol/methanol can be obtained. Plus, woods (from which methanol was obtained in
the past) dont grow fast enough to provide enough methanol... while rape/soy plants do.
Anyway... check this
http://www.agdepartment.com/RES/Chris%20Zygarlicke.pdf
There is alot of interesting info on biodiesel and ethanol... comparing the feasibility of each.
What I fail to understand is why most people are so reluctant to accept that biofuels are valid alternatives - just think this: If the amount of money
that was invested into petrol chemistry and its rationalisation was invested into renewable fuels, then clearly renewable energies would win over - at
least once conventional petrochemical fuels would run out (but then the investment would occur by necessity).
But frankly, it disgusts me. How can anyone claim that CO2 that wasnt present for millions of years, and is suddenly pumped into the atmosphere within
the past 50 years, does not have any effect on our planet (like some morons in the American administration)? This 'insight' (which it is not
really, as it is obvious) should be more than enough for governments to enact laws that biofuels or alternative energies should be heavily funded, and
even tax-relieved to encourage the individual to use them... (and guess what, this is what happens in Germany already, with solar power... but it
seems like the rare front-runner)
but I am babbling away... I guess I just had to get it off my chest! I just hate lack of foresightedness... and we (or my potential kids) and the
planet has to live (or die) with it just because the governments might lose a bit money to invest on friggin military development!
[Edited on 4-6-2004 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Ethanol is already in use in some fuels of certain poor countries (Brasil)...it only needs smal adaptation of benzine motors.
For diesel...you can drive nearly with what you want as soon as it is a fuel with a moderately high flash point..here in Belgium agricultural
"Tracteurs" (kind of trucks for fields and soil work, farms) run with colza oil...
I have a dream that cars run on essential oils...so earth atmosphere would be a big aromaterapy room....menthol, carvone, lavandene, pinene, limonene,
thymol, vanillin,canabissol,....
No more bad smelling exhaust motor fumes...
    
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Nice idea, pity it doesn't work.
Once you burn pinene it isn't pinene any more and it smells of CO2 and water (ideally).
OTOH canabinol cheap enough to use as fuel is an interesting idea.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
No combustion is complete even with catalytic exhaust tube so there will be a smell of the original compound plus some other smel of derivatives ;-)
and decay compounds
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I'm doing many experiments with biodiesel based on ricin oil and ethanol (both cheap and widely available here). However, phase separation does
not seem to ocurr.
How can I test the products for the presence of the esters?
I thought about saponifing the whole thing, than only the esters would remain, hopefully floating in a separate phase, but frogfot said here:http://www.sciencemadness.org/talk/viewthread.php?tid=2282
that even water will hydrolise the esters...
Are the esters that fragile?
Any Idea?
Another question: Has anybody ever heard of a solid (non soluble or fully recoverable when reaction is over) catalyst? Something that hydrolises
triglicerides?
Edit: I was thinking of a very insoluble hydroxide like CaOH in pellets, or citric acid...
Anybody out there interested in biodiesel chemistry?
[Edited on 21-9-2004 by Tacho]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
But frankly, it disgusts me. How can anyone claim that CO2 that wasnt present for millions of years, and is suddenly pumped into the atmosphere within
the past 50 years, does not have any effect on our planet (like some morons in the American administration)? This 'insight' (which it is not
really, as it is obvious) should be more than enough for governments to enact laws that biofuels or alternative energies should be heavily funded, and
even tax-relieved to encourage the individual to use them... (and guess what, this is what happens in Germany already, with solar power... but it
seems like the rare front-runner)
|
Tsk, Tsk, be careful of what you say. Biodiesel is no solution to the CO2 problem, obviously. The only worthwile solution at the moment is hydrogen
produced from nuclear fission energy. Remember that fissioning 1kg of uranium saves you about 800 000kg of CO2 in the atmosphere... (taken into
account both processes have the same efficiency)
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I remember reading somewhere that air bubbles recovered and analysed from the Carboniferous, which began about 300 million years ago, were found to
have 5 times the concentration of CO2 in them as in present-day air (and about 1½ times the present O2 concentration). At that time, and for most of
Earth's geological history since then until only about 30 million years ago (Miocene, when Antarctica started to become glaciated), temperatures
have usually averaged about 22ºC, compared to 15ºC now, and 7ºC at the height of the Pleistocene ice age (2 million to 12,000 years ago). There is
no chance of atmospheric CO2 levels returning to what they were in the Carboniferous, because of the sequestration of enormous quantities of CO2
mostly as carbonate rocks since then, especially during the Cretaceous.
John W.
[Edited on 21-9-2004 by JohnWW]
|
|
|
Bio
Harmless

Posts: 5
Registered: 21-9-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by JohnWW
I remember reading somewhere that air bubbles recovered and analysed from the Carboniferous, which began about 300 million years ago, were found to
have 5 times the concentration of CO2 in them as in present-day air (and about 1½ times the present O2 concentration). At that time, and for most of
Earth's geological history since then until only about 30 million years ago (Miocene, when Antarctica started to become glaciated), temperatures
have usually averaged about 22ºC, compared to 15ºC now, and 7ºC at the height of the Pleistocene ice age (2 million to 12,000 years ago). There is
no chance of atmospheric CO2 levels returning to what they were in the Carboniferous, because of the sequestration of enormous quantities of CO2
mostly as carbonate rocks since then, especially during the Cretaceous.
John W.
[Edited on 21-9-2004 by JohnWW] |
And the planet was covered with green material (lots of it). I read a quote Scientific American I believe, that a it would take the green material
(soybean?) on a 40 acre field to make ONE gallon of gasoline.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Tacho
How can I test the products for the presence of the esters?
|
They are BOTH esters.
| Quote: |
Another question: Has anybody ever heard of a solid (non soluble or fully recoverable when reaction is over) catalyst? Something that hydrolises
triglicerides?
|
LIPASE.
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The hydroxyl group in ricinoleic acid will tend to make the phase separation less effective.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
I bought this medicine which is basically pig’s pancreatin. It’s made by Solvay Farma (local branch of Solvay Pharmaceuticals GmbH – Germany).
Each pill should have 6.500 FIP units (?) of lipase.
I mixed one crushed pill with 10ml of soy oil and nothing happened overnight.
The references I got (chinese and american) say they used lipase from fungus, imobilized in acrilic resin. I read in the box that my pig’s lipase
only cleaves fatty acids in 1 and 3 position, still, nothing, really nothing, seemed to have happened to my soy oil.
I’ll try calcium acetate + barium acetate (United States Patent 5,525,126) if they form an insoluble pellet. Maybe mix with, say, portland cement.
As for testing, I’ll have to develop TLC techniques, or some specific chromatografic technique.
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
| Quote: |
I bought this medicine which is basically pig’s pancreatin. It’s made by Solvay Farma (local branch of Solvay Pharmaceuticals GmbH – Germany).
Each pill should have 6.500 FIP units (?) of lipase.
I mixed one crushed pill with 10ml of soy oil and nothing happened overnight.
The references I got (chinese and american) say they used lipase from fungus, imobilized in acrilic resin. I read in the box that my pig’s lipase
only cleaves fatty acids in 1 and 3 position, still, nothing, really nothing, seemed to have happened to my soy oil. |
The fact is that the room temperature is not the optimum temperature of the enzyme, and it will take ages before any noticeable effect. If I remeber
correctly the immbolized enzyme has an optimum temperature of approximately 45 deg celcius, while the free enzyme has a lower optimum temp of around
33 deg celcius - internal body temp (This reduction in temp is due to the stabilty of the enzyme. In the immobilized state the structure is far more
stable.) The optimum pH is of around pH7, but it functions well up to pH8.
Therefore you will require some warming for the rxn to work. Simply heating the container in a water bath will not do, since this could denature the
enzyme. I would suggest an aquarium heater as a controlled heating soure.
Theory guides, experiment decides.
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
Thanks,
I warmed the tube for 3 hours at 45ºC with no apparent results.
Either: 1) The products are an homogeneous mix that looks like the reactants; 2) Some specific condition is needed for the reaction to proceed (some
gastric fluid maybe). 3) The medicine is a scam.
I'm working on a chromatographyc method to check my results with other catalysts.
When I work with methanol+ricin oil+KOH, I get separate layers easily. The same mix with soy oil is hard to tell, since the reactants have two layers
that have to be kept stirred anyway.
I gave up the acetate salts idea. They will dissolve (partially) in the reactants and will pose the same isolation problems that hydroxides have.
|
|
|
| Pages:
1
2
3
..
5 |