Cloner
Hazard to Others
  
Posts: 150
Registered: 7-12-2004
Member Is Offline
Mood: apocalyptic
|
|
cause of explosion
I had a small explosion today in a heat degradation reaction. I was heating by flame about 10g of polylactic acid melt together with 1,4 g zinc powder
and 0.3 g zinc chloride. There was a lot of gas formation in the test tube I used. I was collecting the gas distillate in a corked erlenmeyer with gas
outlet. Fortunately I wasn't hit, although the fume hood was splattered with glass and the usual viscous, dark mess.
When the explosion occured, there was air intake into the heated test tube caused by a pause in heating (I needed to prevent foaming). The fragments
of the blast indicate the top of the test tube exploded. There was quite some distance between the gas outlet of the collecting vessel and the flame
and the test tube was corked and airtight.
I understand that hydrogen may form from the use of zinc powder. Also there is the formation of lactide, a semi high boiling organic compound I was
intending to collect. Now I wonder what could be the cause of the explosion. I don't think it was the hydrogen itself, or the flame. Is it possible
the metal particles caused the gases to react with oxygen?
The lactide molecule looks like this:
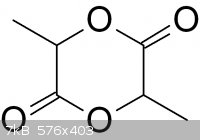
Lactide
[Edited on 9-6-2011 by Cloner]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Cloner  | | I had a small explosion today in a heat degradation reaction. I was heating by flame about 10g of polylactic acid melt together with 1,4 g zinc powder
and 0.3 g zinc chloride. There was a lot of gas formation in the test tube I used. I was collecting the gas distillate in a corked erlenmeyer with gas
outlet. Fortunately I wasn't hit, although the fume hood was splattered with glass and the usual viscous, dark mess. |
Oooo Nooo You have recreated the Wolffenstein-Böters
Reaction, which has not been done since it was banned in 1405.
Nostradamus clearly predicts that the second time it is done
the End of All Time will soon follow!
It is time for the collective to get down of their bended knees
and pray before their periodic tables and ask too be saved.
You have been warned.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Well... hydrogen and oxygen seem like the most likely immediate cause of explosion. A wide range of ratios are explosive. So if your test tube had a
bunch of hydrogen in it and some air entered via suckback, that would create the potential for what you describe. But the initiation is still a
mystery, as the autoignition temperature of H2+O2 is actually rather high.
|
|
|
Cloner
Hazard to Others
  
Posts: 150
Registered: 7-12-2004
Member Is Offline
Mood: apocalyptic
|
|
Wolffenstein clearly described a blackish compound in his 1405 publication but has not provided a quantitative analysis and a molecular formula.
Therefor it is doubtful this is the same result.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
I was under the impression that the Wolffenstein-Böters reaction is an organic reaction converting benzene to picric acid by a mixture of aqueous
nitric acid and mercury(II) nitrate. I thought Wizard Is In was just being cryptic.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Cloner
Hazard to Others
  
Posts: 150
Registered: 7-12-2004
Member Is Offline
Mood: apocalyptic
|
|
Too cryptic then because there is no apparent link to the reaction I describe.
|
|
|