IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
Cheap platinum jewelry as anodes for DIY chloralkali cell?
I am trying to build a home chloroalkali cell to make a small quantity of perchlorates.
I've already converted an old computer PSU into a power supply for the cell, and obtained some KCl.
I've bought titanium mesh for the cathodes and am looking for some platinum plated metals for the anodes. It seems my options are to:
1) Buy purpose built mesh Pt-plated anodes
2) Do my own Pt electroplating
3) Find Pt-plated items from other sources
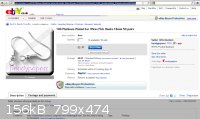
Checking on Ebay, it seems that one possible cheap source of Pt-plated items is cheap jewelry. E.g. see attached picture (put up as a permanent
screenshot rather than a temporary link so that it won't expire once the bidding closes).
The only problem with this is that it would be hard to construct a large surface area anode from a collection of these. The conductive joints between
discrete items would be subject to corrosion if they are made of anything but a noble metal.
|
|
|
Mixell
Hazard to Others
  
Posts: 449
Registered: 27-12-2010
Member Is Offline
Mood: No Mood
|
|
It looks good, maybe too good to be true.
Even if its only plated, 50 pairs for less than 7 bucks?
Even if they were made from aluminium or stainless steel (and plated with nothing) I would consider 100 units of those for 6.5$ to be a bargain.
If we can get proof that this is really platinum plated jewelery, I would gladly order some myself.
And even more, maybe we could ask the seller if he can get his hands on some dirt cheap, platinum plated anodes (seems like he deals quite a lot with
platinum plated jewelery).
|
|
|
IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
It may be so cheap because the plating layer is very thin. But thin is OK for our purposes, especially being so cheap as to be effectively disposable.
Beauty being skin deep, excuse the pun 
But yeah, the price is very low. Their shape is awkward to use as anodes but I was thinking about either straightening them out using pliers, or
having quite a shallow cell running at a high starting pH to quickly absorb any evolved chlorine gas.
|
|
|
IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
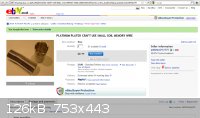
Here's some platinum wire for jewelry in a more suitable form. I've ordered some and will let you all know how my tests go  . The titanium cathodes are being ordered from overseas so I'll try to use platinum
anodes/cathodes while waiting for the cathodes to arrive. . The titanium cathodes are being ordered from overseas so I'll try to use platinum
anodes/cathodes while waiting for the cathodes to arrive.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | I've ordered some and will let you all know how my tests go.
|
An anode for perchlorates for less than a quid???
It's pie in the sky . . .
Reading up on electrolysis might be more productive!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Last time I was looking for some platinum for an electrode in potentiometric titrations I saw these ‘Beadypapers’ allegedly platinum coated ear
wires and thought: ‘naff off, ain’t no Pt there!’
It’d be interesting to see your results. Try dissolving some in Aqua Regia, you’ll soon know whether there’s any Pt there…
I ended up spending £15 for for 3 cm of real platinum wire (not coated).
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Platinum plating
See pages 1579-1580 in Brauer's: cis-dinitrodiammineplatinum (II). This salt is THE salt for platinum plating on titanium and other
substrates. Industrially, we call it "P" salt. Most important thing is preparation of the substrate.
I've prep'd it many times, and it's remarkably easy to make and use.
See attachment for details on plating.
Attachment: platinum plating.pdf (8kB)
This file has been downloaded 858 times
[Edited on 16-4-2011 by Fleaker]
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
Where can I buy enough of this salt for under USD300 to make about two 150x30mm titanium mesh Pt-plated electrodes? That was the quote I got to get
two custom 150x30mm platinum plated mesh electrodes shipped from USA.
The closest thing I could find through my usual suppliers in the UK are:
http://www.atomscientific.com/products.php?product=456
Cisplatin (cis-Dichlorodiammineplatinum(II))
£39.40 for 100mg
http://www.atomscientific.com/products.php?product=582
Carboplatin (Diammine(1,1-cyclobutanedicarboxylato)platinum(II))
£46.60 for 25mg
http://www.lab-shop.co.uk had tons of hits of different "platinum" forms in the search.
In true eccentric fashion is it feasible to scavenge platinum from old catalytic converters (e.g. aqua regia on the Pt-coated ceramic bits?)
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
If you're in UK, go to Johnson Matthey. They will sell it.
I'd offer to post you some, but I'm across the pond.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Contrabasso
Hazard to Others
  
Posts: 277
Registered: 2-4-2008
Member Is Offline
Mood: No Mood
|
|
http://www.etltd.co.uk/contact.html are electrode suppliers in the UK. See if thy have what you want. Otherwise someone on APC forum was selling
some OK lead dioxide anodes, and the cathodes to match them.
If you want to go from chloride to perchlorate start with a lead dioxide anode anything else will fail
|
|
|
IndependentBoffin
Hazard to Others
  
Posts: 150
Registered: 15-4-2011
Member Is Offline
Mood: No Mood
|
|
Hi guys, I just got the platinum coils today from Ebay and ran them in a saturated NaCl solution at 5V off an ATX PSU. They certainly looked platinum
plated as I've bought white gold jewelry for some close women before, and the white shine was just like how I remembered it.
Unfortunately within about ten seconds of using it as an anode, the white metallic sheen was lost very quickly and a greenish solution (probably the
Ni substrate being oxidised to NiCl2, as the wire was described as a shape memory wire which usually has nickel in it) started appearing around it.
The Pt plating must either be very thin or with lots of surface defects, hence allowing the evolved chlorine to attack the Ni base.
So it looks like these DIY anodes won't work 
[Edited on 18-4-2011 by IndependentBoffin]
|
|
|
crystalXclear
Harmless

Posts: 28
Registered: 30-12-2010
Member Is Offline
Mood: No Mood
|
|
ANYTHING FROM EBAY WILL PROB BE SH1TE, proffit proffit profit, grubby little grabbers.
If you give me till this afternoon, (it's my birthday so i'm buisy this morning) I can let you know where to get the best deal on plat in the UK. I'm
a lapidaryst & jeweller by trade (30+ yrs), I get my plat from the states as part of a large multi precious metal order, but not that offten.
what's the rules on posting company names? it doesn't look to be an issue, just checking is all.
Out of interest, How many ppl would be interested in plat, palladium etc as pure metals, plating salts, solutions, anodes, so on & so forth?
Xtal
|
|
|
crystalXclear
Harmless

Posts: 28
Registered: 30-12-2010
Member Is Offline
Mood: No Mood
|
|
Johnson Matthey for the UK
RIO GRANDE in the States, WoW, yer gunna love that site, She's My one & only
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
probably the cheap Pt plated thing , is cheap Pt plated thing , in other word if you paid more you should get wath you want.
I never asked for this.
|
|
|
crystalXclear
Harmless

Posts: 28
Registered: 30-12-2010
Member Is Offline
Mood: No Mood
|
|
they just dangled the ear wires toe's in the plating solution. lol
Theres allways the plat wire from those alternative hydrogen energy site's,
I have a NOS fuel tank/cylinder, about 3gal (GB), It said it was solid plat as far as they knew. It has (PTxxxx) something scratched onto the side of
it, what are the hances of it being solid?
|
|
|