Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Accelerated corrosion?
Hello,
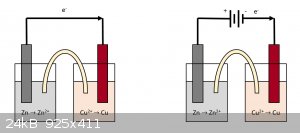
when building a simple voltaic cell, made of two common electrodes in a solution of their ions (let's take Cu/Cu2+ half cell coupled with a Zn/Zn2+
half cell), and we close the circuit, electrons will flow from the half-cell with lower potential (Zn/Zn2+) to the one with higher potential
(Cu/Cu2+), effectively leading to the corrosion of Zn and reduction of Cu2+.
This reaction will have its own rate of reaction and once the Zn electrode is consumed, the voltaic cell is fully spent.
What I was wondering was: what would happen if we connected a battery in series with this voltaic cell ? Would the extra potential accelerate the
consumption of the Zn electrode, effectively accelerating the rate of corrosion of Zn? Or that extra potential would not affect this rate of reaction?
Would this be equivalent to shorting the battery, or the voltaic cell still somewhat behaves like a resistance?
In my head I pictured the extra potential "helping" push the electrons across the two half-cells, therefore accelerating the rate of reaction. On the
other hand, I'm inclined to think that the increased potential difference would only affect the thermodynamics of the process but not the kinetics.
I drew a sketch of the two different systems
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Depends on how you connect it. With the positive of the cell connected to the positive of the battery.
This is the same as connecting 2 batterys in parrallel, their. Charges will flow current and attempt to equalize. With the positive of the battery
connected to the negitave of the cell, your battery/cell becomes the load. Maxium current will be drawn and your battery and cell will discharge
[Edited on 19-1-2024 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rainwater  | Depends on how you connect it. With the positive of the cell connected to the positive of the battery.
This is the same as connecting 2 batterys in parrallel, their. Charges will flow current and attempt to equalize. With the positive of the battery
connected to the negitave of the cell, your battery/cell becomes the load. Maxium current will be drawn and your battery and cell will discharge
[Edited on 19-1-2024 by Rainwater] |
Why would it be equal to 2 batteries in parallel? Shouldn't it be in series, for how I drew it?
And also, what I wanted to know was if putting a battery in series and increasing the total potential would have increased the rate of the redox
reaction.
[Edited on 20-1-2024 by Metallus]
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
I always get confused with potentials in cells, conventional, non conventional currents. Give me a headache. So i wrote what would happen under both
conditions. In your drawing, Assuming the red electrode has a positive potential. Your drawing shows the negitave of your cell connected to the
positive of your battery. Using Kirchhoff's circuit law to analize the circuit you will discover that current will flow in this circuit as long as one or both of your voltage
sources are non-zero.
I think this will increase the reaction rate, resulting in the zinc electrode being consumed quicker.
By reversing the connection KCL shows us that the greater voltage potential will flow current into the other one. Being batterys this will result in
one discharging while the other charges. If the battery has a greater potential than your cell, electrolysis will result, potentially recharging your
cell
Edit:
If you try to calculate the currents you will need to include the parasitic resistance of both devices
[Edited on 21-1-2024 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
Metallus
Hazard to Others
  
Posts: 116
Registered: 16-5-2013
Member Is Offline
Mood: No Mood
|
|
Thank you!
Yes, the second case is what happens in electrolysis, where you spend energy to make non-spontaneous reactions happen (like when you want to reduce
otherwise irreducible species like Li+, or electroplate something or to recharge batteries), but in this case I was wondering what would have happened
in the other case, where you double down on the potential to further accelerate an already spontaneous reaction.
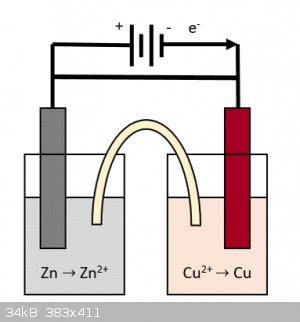
About the positive/negative connections in my drawing, the Zn anode is where electrons are produced and sent to the Cu cathode which happily accepts
them to reduce the Cu2+ to Cu. So the positive end of the battery should pull those electrons "better" and accelerate them towards the Cu electrode
which should be positively charged, that's the intention. I also get confused with the conventional currents directions, that's why I'm speaking in
terms of electrons moving (the conventional current would have the opposite sign otherwise).
In such case, the schematic would be equivalent to two batteries connected in series with no apparent resistance, so this "device" should just
discharge at a rate dictated by.... by what? In my head, it's like I'm shorting these 2 batteries, but maybe the redox rate is so slow that rapid
discharge and heating up is avoided.
Or maybe, since the ions concentrations at a given time depend on the amount of electrons (and therefore of charge) that has flown in the circuit, the
rate of reaction is more dependent on the current intensity rather than the total voltage.
In this case, would connecting the battery in parallel increase the current intensity in this circuit? Or would this just result in the battery
bypassing the voltaic cell and just shorting itself?
[Edited on 22-1-2024 by Metallus]
|
|
|