| Pages:
1
2 |
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
Sulfuric acid purification problems
While I usually buy the Walmart Kleen-Out brand drain opener, I decided to try the Ace Hardware Rooto stuff because the msds seemed to indicate a
higher purity. Instead of the nice clear liquid I was expecting, it was cloudy with some white precipitate.

After running it through a filter it came out clear:

I added some hydrogen peroxide and brought it to a simmer. I couldn’t heat it for long because it got dark. I did notice that it turned yellow but
reverted to clear as it cooled. After it cooled I measured the density and found it was only about 94%. I guess it wasn’t boiled long enough. I
tried again today and this time I boiled it longer. But when it cooled, a precipitate formed.

What’s going on? The only thing I can think of is that there’s a water soluble substance that precipitated when the acid got more concentrated.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Quote: Originally posted by Sir_Gawain  |
What’s going on? The only thing I can think of is that there’s a water soluble substance that precipitated when the acid got more concentrated.
|
nailed it.
you can try to collect and analyze the precipitate to see if it will interfere with your chemistry, or distill it to remove the contamination.
"You can't do that" - challenge accepted
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
Distilling sulfuric acid is not something I feel comfortable doing. I’m afraid my condenser’s going to explode.
I have no idea what the precipitate is; it can’t be organic or it would’ve been destroyed by the heat and peroxide. I do remember reading
somewhere here that someone had a crystalline material appear after distilling drain opener, so maybe it’s the same thing.
Edit: I just checked the density and it’s about 97% sulfuric acid.
[Edited on 16-11-2023 by Sir_Gawain]
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Some time ago I mentioned a problem that I had with Rooto sulfuric acid. I always wash my hexane with sulfuric acid to remove some initial impurities
before distillation. I have always used sulfuric acid from Duda for this purpose. However, I had some of the Rooto acid to use up so I used it
instead. Shaking Rooto acid with the tech hexane gave an emulsion that took some time to separate which had never happened with the Duda acid. To get
to the point, I suspect that the Rooto acid contains some sort of detergent like material which is what you are seeing as a contaminant. There may be
no practical way to get rid of it.
Just my long winded 2 cents worth.
AvB
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
Was your acid cloudy to begin with?
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Achilles
Harmless

Posts: 24
Registered: 18-9-2023
Member Is Offline
|
|
it is normal in every sulfuric acid you buy it will turn yellow if heated and clear when cool, the only way to get rid of it is distillation..
extremely slow and the distillate will get water from the air so its complicated, unless you need it for tritrations it still work well just for
nitric acid youll get a 80% since it will oxidize the impurities and get the water from it
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
You can try and freeze it out using an ice bath.
Melting point is about 10c.
"You can't do that" - challenge accepted
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
I had no idea the melting point was so high. I've cooled sulfuric acid in a freezer to -18C and it's never frozen. Do you think that 2% of water
lowers it that much?
[Edited on 16-11-2023 by Sir_Gawain]
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Just a couple of quick notes regarding distillation of sulphuric acid:
The scariest bit of chemistry I've done for a long time.
Bumping is a major hazard - boiling hot concentrated sulphuric acid will destroy anything organic, skin, eyes etc.
No chance of remedial actions no matter how quickly you act.
If you do distill, then collect and keep all of the fractions... all useful.
No need for peroxide or other additives.
Cheap Chinese borosilicate glassware is more than adequate.
.......................................
I've had success and failures (bumping) using boiling stones,
If I do another distillation I will try air via an aquarium air pump, drying tube and a glass tube drawn to a capillary to produce a stream of tiny
bubbles.
In Malaysia I can buy Reagent or Analyytical grade acids via a home shopping sites, so I will probably not distill sulphuric acid again.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
If you want to know more with great detail get some (good soul) to get you this
https://pubs.acs.org/doi/pdf/10.1021/ja01141a010#
Yob
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Sir_Gawain  |
I had no idea the melting point was so high. I've cooled sulfuric acid in a freezer to -18C and it's never frozen. Do you think that 2% of water
lowers it that much?
[Edited on 16-11-2023 by Sir_Gawain] |
Yes, that little bit of water drops the freezing point significantly.
|
|
|
leau
Hazard to Others
  
Posts: 122
Registered: 3-12-2021
Member Is Offline
|
|
Four more documents including that mentioned up thread are in the attached archive 
Attachment: SulfuricAcid.zip (5.5MB)
This file has been downloaded 203 times
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
Thanks, leau! I wonder, would be possible to purify sulfuric acid by freezing it? While it probably wouldn't raise the concentration much, it might
exclude soluble contaminants.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
Ok, so I scraped the precipitate off of my filter and it’s not soluble in water.It actually settled out pretty quickly, which makes sense given
water’s lower density and viscosity than sulfuric acid.

“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Flame test.
Found this while googleing
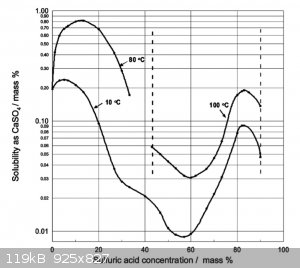
solubility of CaSO4 in H2SO4
Fits the observations so far.
Insoluble in water
White powder
Test:
It shouldnt react with any mineral acid, sulfuric, nitric, HCl
Heat a sample red hot. Let cool, crush into powder
add a few drops of water.
If it sets rock hard you for CaSO4.
[Edited on 16-11-2023 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
Calcium sulfate was also my first guess. Are calcium compounds used at any point during the manufacture? Perhaps some CaO was used to clean up and got
in it.
I could try dissolving some CaSO4 in sulfuric acid and seeing if it behaves the same way.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Online
Mood: No Mood
|
|
Quote: Originally posted by Rainwater  |
Heat a sample red hot. Let cool, crush into powder
add a few drops of water.
If it sets rock hard you for CaSO4.
[Edited on 16-11-2023 by Rainwater] |
If you are trying to make gypsum plaster you don't heat it to red heat.
That will convert it to anhydrous stuff and that doesn't react with water.
You need to heat it somewhere about 200 or 250C, I think.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
I’ve filtered about a liter of it and don’t have nearly enough to try this test.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
Next time I filter some I'll try it. By the way, 24 hours later and the precipitate is almost completely dissolved into the water. It's gotta be
calcium sulfate.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Cathoderay
Hazard to Self
 
Posts: 54
Registered: 29-1-2023
Location: US-Texas
Member Is Offline
|
|
Manufacturers mix additives to sulfuric drain cleaners (inhibitors) so that it is less corrosive to metal pipes (cast iron, etc.). It isn't so much a
impurity of manufacture situation, although for drain cleaning use the impurities in the raw acid don't matter much.
The inhibitors could be organic or inorganic.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
Any organic inhibitors would have been destroyed by boiling with hydrogen peroxide. I don't know of any inorganic inhibitors, but that's probably what
this is.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
To test for calcium sulfate, I added a solution of sodium carbonate to the beaker I had dissolved the precipitate in. A brown solid immediately
formed.

Not what I expected.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Cathoderay
Hazard to Self
 
Posts: 54
Registered: 29-1-2023
Location: US-Texas
Member Is Offline
|
|
Seems like "Out of the frying pan and into the fire."
That seems like that is a lot or precipitate, much more than the amount of inhibitor I would expect.
What exactly did the MSDS say?
How much hydrogen peroxide (compared to the acid) and what was the concentration?
Maybe you didn't completely destroy the organic component.
Are you sure the filter was clean?
The sodium carbonate pure?
Calculating the percentage based on the density only works if it is only acid and water.
Calcium sulfate might work as an inhibitor.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 420
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Like a pendulum
|
|
1. There's not as much there as it looks like; it's really low density.
2. The MSDS says 93% sulfuric acid and 7% water. It makes no reference to any inhibitors.
3. I added about 25 mL of 34% hydrogen peroxide and boiled it for over 30 minutes.
4. I ran some piranha solution through it before filtering the sulfuric acid. It's fairly new and I haven't used it for anything that could get stuck
in it.
5. The sodium carbonate was washing soda from the grocery store, which is pretty pure.
6. Calcium sulfate is my best guess, but judging by the brown color that's not all. Maybe iron?
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Texium
|
Thread Moved
20-11-2023 at 06:23 |
| Pages:
1
2 |