| Pages:
1
2
3 |
littlesky
Harmless

Posts: 22
Registered: 12-6-2023
Member Is Offline
|
|
A powerful,unreported new primary explosive--NiCC
Preface:Before introducing NiCC,let's first introduce a primary explosive that someone may know.
Nickel tricarbohydrazide perchloric(NiCP) is a less well-known primary explosive.But its excellent performance has attracted the attention of many
Chinese enthusiasts.Powerful,easy to prepare,and environmentally friendly are its advantages.According to different preparation conditions,this is a
kind of powder from light blue to dark blue.(Annex 1)
However,raw materials-perchlorate(especially sodium salt and ammonium salt)or perchloric acid are almost impossible to obtain and expensive.And its
theoretical sensitivity is not high,but due to the presence of numerous edges and fractures in the crystal,its actual sensitivity is very high,
similar to that of peroxide HMTD.(Annex 2)
About a year ago, when I was doing a test,I accidentally found that the mixture of nickel tricarbohydrazide nitrate and Potassium chlorate had strong
explosive power.So, would it have a better effect if chlorate ions entered the molecule instead of simply mixing?
I have consulted many similar article,but there are no results.Only one article mentioned the Copper(II) chlorate complex Therefore, I believe that
this compound and similar substances have not been synthesized by other researchers.
Later,I successfully synthesized this substance through experiments. After testing,I found that it indeed has strong detonation ability. Sensitivity
similar to NiCP but lower.And after multiple experimental optimizations,more suitable preparation conditions were found.
Warning:This energetic material has not undergone strict sensitivity testing, and there is no data to prove its sensitivity.
After my simple testing, I believe that its sensitivity is lower than NiCP, but some people have also made dangerous high sensitivity products. So it
is not recommended to prepare in large quantities.
1.Preparation
Source of chlorate:I prepare it through Sodium chlorate.In China, Chlorate is difficult to obtain,but Sodium chlorate can be purchased as a water
treatment agent(called COD).You can also get a more pure nickel chlorate solution by mixing Barium chlorate and Nickel(II) sulfate solution,filtering
out Barium sulfate.
Nickel(II):I use Nickel(II)nitrate hexahydrate. After many experiments,I found that the solubility of nickel tricarbohydrazide nitrate mentioned above
is very high.After the experiment of other enthusiasts, Nickel(II) sulfate hexahydrate is also feasible.
Carbohydrazide(CHZ):a chemical raw material that can be purchased directly.
Let's start preparing now
Weigh 0.79g CHZ and 1g NaClO3, and dissolve them together with 4ml of water.(No reaction,no need to worry)
Also weigh 0.85g Ni(NO3)2•6H2O and dissolve in 4ml of water.
Freeze 10ml of distilled water at the same time for subsequent washing.
Take Nickel(II) nitrate solution as the bottom solution, and drip Sodium chlorate CHZ mixed solution under magnetic stirring,and the solution turns
blue.
Wait for a while, a small amount of sediment appears
Place the beaker in an ice bath(-5~-10℃)and stir continuously.At this time,a large amount of precipitates will precipitate and settle to the
bottom.The upper clear liquid is light blue.
Place the beaker in the freezer to further cool down to increase yield. After filtration or suction filtration,wash with cold water 2-3 times.It is
recommended to use suction filtration here,because this material has a large solubility and a fast dissolution rate,and can quickly reach the
precipitation Solubility equilibrium when the temperature is slightly higher.Just ordinary filtration time can cause a significant loss of product.
It is difficult for the yield to exceed 80% during ordinary filtration.After preparing so many times,the highest yield is only around 78%,while
suction filtration is estimated to exceed 85%
Dry naturally to obtain blue powder
This is the final product(Annex 3)
2.Test.
Mechanical sensitivity:After some testing,I feel that it is lower than the nicp,and capable enthusiasts can help me measure the specific data.
flame sensitivity:It can be directly ignited and detonated by KNSU charges(KNSU:60~65%Potassium nitrate,40~35%Sucrose),but this is unlikely to happen
in NiCP.
Power test:Accurately weigh 5mg NiCC,Aluminum foil wrapping,fuel ignition.(Annex 4)
  
|
|
|
littlesky
Harmless

Posts: 22
Registered: 12-6-2023
Member Is Offline
|
|
Annex 4
Attachment: 5mgNiCC slow.mp4 (2.5MB)
This file has been downloaded 503 times
|
|
|
underground
National Hazard
   
Posts: 715
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
What is the storage stability ?
|
|
|
littlesky
Harmless

Posts: 22
Registered: 12-6-2023
Member Is Offline
|
|
Of course it's safe.I synthesized this compound on June 22, 2022 and it has been stored at room temperature for over a year. So far, there has been no
change in color or morphology. The explosion test is also quite perfect. In addition, I also prepared a solution of this compound and stored it in
sunlight and in a dark place for one month without any color change. It should be possible to demonstrate the long-term storage stability of this
substance
 
|
|
|
underground
National Hazard
   
Posts: 715
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Cool. The copper/calcium/magnesium nitrate salt of CHZ could be a good secondary too
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 443
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Does anyone know of a commercial source in USA of carbohydrazide/semicarbazide? Or of NaNO2 for that matter? I can only find it direct through the
Chinese for last couple of years.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 493
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Still in 2022
|
|
Sodium nitrite can be bought from sporting goods stores as bait cure. I bought mine here. They're out of stock right now but they restock fairly regularly.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
littlesky
Harmless

Posts: 22
Registered: 12-6-2023
Member Is Offline
|
|
Haha, chz complexes are very interesting. I have been studying copper complexes with chz for a year. Nitrates have various isomers. But without
exception, they are very weak and unstable.
According to different preparation methods (temperature, solvent, etc.), dark brown, dark blue and Baby blue powders may appear. The dark blue isomer
turns yellow and then green after less than a month of storage. Baby blue isomers will decompose spontaneously and slowly. The black isomer is
relatively stable and has a high density, but the preparation conditions are very strict.
These three isomers cannot continue to burn on their own. Only when continuously under the flame can it burn, emitting a green flame. When heated and
decomposed, the two blue isomers first melt and then burn and explode. It is obvious that melting is endothermic and cannot sustain combustion on its
own. This makes it impossible for this substance to be applied in the field of primary explosives.I have tried to detonate copper nitrate of CHZ, but
failed.
I also made Chlorate and Perchlorate of chz copper complex. The former is a Dangerous goods that is easy to self explode (which almost kills me), and
the latter is an explosive with high sensitivity and cannot be used in the field of primary explosives.
|
|
|
Vpatent357
Harmless

Posts: 22
Registered: 10-7-2014
Member Is Offline
Mood: No Mood
|
|
Synthesis of a new primary explosive:Nickle tricarbohydrazide perchlorate(NiCP)

Video YT 
Small blasting caps, fingers safe!
|
|
|
littlesky
Harmless

Posts: 22
Registered: 12-6-2023
Member Is Offline
|
|
I made this video 
|
|
|
underground
National Hazard
   
Posts: 715
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
What is the difference between the chlorate and perchlorate salt ?
Have you ever tried other complexes of CHZ with other metals like magnesium calcium cobalt etc
[Edited on 17-7-2023 by underground]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1447
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Difference can be advantage use NaClO3 which is easily avaliable (produce) than NaClO4. But carbohydrazide availability as key precursor can be
problem in a lot countries.
A like every new substance, also Nickel carbohydrazide chlorate (NiCC) require perpedicular test 1000 mg against lead brick or aluminium brick.
The test on a can of coca cola has no telling value.....
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
underground
National Hazard
   
Posts: 715
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
littlesky have you ever tried to nitrate CHZ ? Diaminodinitrourea may be formed
[Edited on 18-7-2023 by underground]
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I have experimented a little with the perchlorate variant. I found that preparation was easy and high yielding (89% with no effort to maximize yield
except for using ice water to wash the product). NICP is a sky- or baby blue material. It can be air dried and doesn't seem hygroscopic, though I
haven't tested that rigorously. If contacted by a flame when on a spatula, it sputters and flashes, but seems less inclined to take fire than NAP or
lead styphnate.
If contained in a fold of aluminum foil and held over a flame, it detonates extremely violently, perhaps even more so than NAP. It DDTs in amounts as
low as 5 mg from the spit of a thin (1.5 mm) BP fuse when compacted into a piece of PVC tubing, with the two ends closed with compacted Al-foil and
tissue paper respectively.
I have tested sensitivity with my oblique impact machine, which combines impact and friction testing by striking the sample at a shallow angle with a
steel pendulum (see attached picture). The sample is spread evenly on a small piece of 100 grit sand paper and clamped to a steel plate on the base of
the apparatus. Then the pendulum (550 g) is raised to a certain height and released. This is repeated 5 times at a given height and the number of
positive tests is recorded.
For NICP 90 cm (4.86 J) gave 2/5 (2 go and 3 no go the positives are number two and three from the bottom) while 95 cm gave 1/5. For comparison, NAP
gave 2/5 at 110 cm.
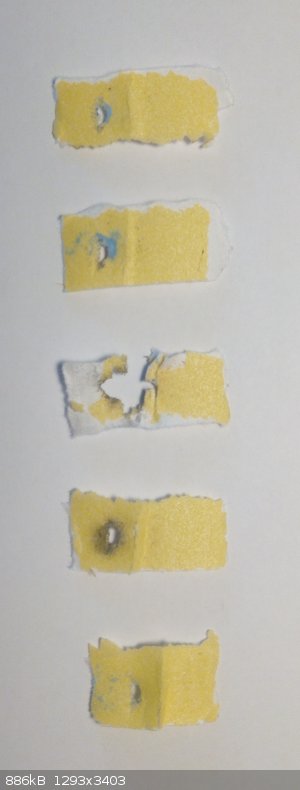

|
|
|
MineMan
International Hazard
    
Posts: 1030
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Just can’t beat NAP. Nor would I expect one too. Aminoguanidine is a very good and stable molecule.
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Well, I'm not quite certain about that. It is true that NAP is slightly less sensitive, but both of them are very insensitive for primaries. As I see
it, NAP has the disadvantage that due to the air sensitivity of free aminoguanidine, it is destroyed by contact with liquid water. NICP has the
disadvantage that carbohydrazide is not as easy to find or prepare.
I am thinking of experimenting with some other combinations of ligands and (transition) metal ions to see if I can find other promising materials (as
other members have too).
|
|
|
underground
National Hazard
   
Posts: 715
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
I am pretty sure most Ni chlorate/perchlorate complexes are primaries. CHZ can be bought from Alibaba at relatively good price. 2kg of CHZ would be
enough lifetime supply for expiramenting.
CHz is a nitrogen rich compount, i believe other insensitive CHZ complexes would be good secondaries too. Maybe magnesium copper calcium nitrate
complexes.
[Edited on 26-7-2023 by underground]
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I prepared the copper analogue to NICP. It is a very visually pleasing reaction yielding a beautiful midnight blue product. I have only tested its
reaction to flame and indirect heating, and in these tests it doesn't seem very impressive. Of course, that is hardly conclusive.
|
|
|
underground
National Hazard
   
Posts: 715
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Try to detonate a few grams and compare it with ETN.
Magnesium complex in theory will outperform all the rest complexes due to the low molar mass of magnesium metal compared to other metals.
[Edited on 26-7-2023 by underground]
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
It will be some time before I have an opportunity to test anything else, but yes, more experiments with the copper complex will be undertaken.
Magnesium is not great at making complexes with nitrogen rich ligands. It has an affinity for hard oxygen ligands such as water or hydroxylates. For
this reason I think transition metals such as iron, zink or cobalt are much more promising.
|
|
|
underground
National Hazard
   
Posts: 715
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Microtek  | It will be some time before I have an opportunity to test anything else, but yes, more experiments with the copper complex will be undertaken.
Magnesium is not great at making complexes with nitrogen rich ligands. It has an affinity for hard oxygen ligands such as water or hydroxylates. For
this reason I think transition metals such as iron, zink or cobalt are much more promising. |
What about Calcium
|
|
|
Microtek
National Hazard
   
Posts: 920
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Same as with magnesium. It is related to the concept of hard and soft acids and bases, which is in turn related to charge distribution and
polarizability of the metal ion. Small, "hard" metal ions (Lewis acids) such as all of the group one and two elements form stronger complexes with
"hard" bases such as those containing oxygen (eg. water). If you look at the wikipedia article about HSAB theory, you can find an overview of which
metal cations are considered hard and which are soft. This being chemistry, it is a spectrum of hardness rather than definite categories, so
exceptions to these rules do exist.
Nevertheless, this is the reason I think that the transition metals are a better group to look towards for complexation centers in energetic
compounds.
|
|
|
underground
National Hazard
   
Posts: 715
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Interesting. Obviously i am not a chemist and i am still learning.
|
|
|
littlesky
Harmless

Posts: 22
Registered: 12-6-2023
Member Is Offline
|
|
Ok,guys.Do not try to synthesize Chlorate/Perchlorate of chz copper complex, they are extremely unstable dangerous compounds.I once synthesized
Chlorate of chz copper, and this substance blew itself up in the middle of the night
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by littlesky  | | Ok,guys.Do not try to synthesize Chlorate/Perchlorate of chz copper complex, they are extremely unstable dangerous compounds.I once synthesized
Chlorate of chz copper, and this substance blew itself up in the middle of the night |
Did you test and have the same issue with the perchlorate salt? Or are you assuming chlorate and perchlorate will be the same? They will generally
have very different properties.
|
|
|
| Pages:
1
2
3 |