| Pages:
1
2
3 |
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
3-amino-1-nitroguanidine Salts
I have been testing some ANQ salts and so far, haven't found anything spectacular. This thread is intended to house experiments for ANQ salts, both
simple ionic ANQ salts and condensations like nitriminotetrazole etc.
This first salt is an attempt at Cuprammine Perchlorate + ANQ.
The TACP was prepared via LL's jar method.
Tetramine copper perchlorate (still in solution) in excess had ANQ added to it. The reaction was a bit dramatic. It immediately fizzed while the color
changed to brown then purple/pink. The end result is a mass of purple powder. The powder appears to be a slightly more energetic version of TACP. Over
open flame in Al foil confinement there is partial detonation but it is sporadic. I attempted to fuze detonate 750 mg loosely loaded in a plastic hull
and it didn't DDT nor did it burn completely. The most significant impression this made was the dramatic complexation with a visually positive
affirmation that something is complexing but in terms of flame sensitive behavior, I don't see much difference from TACP.
I'm interested in testing salts of condensation products of ANQ. I suspect that it will react with formic acid (analogous to AGu) to form presumably a
Nitro Triazole? Can anyone confirm that makes sense as a formic acid + ANQ condensation product?

The inspiration for these tests was this klapotke article.
Attachment: transition_metal_complexes_of_3-amino-1-nitroguani.pdf (2.2MB)
This file has been downloaded 220 times
[Edited on 13-4-2023 by Hey Buddy]
Update: This lavender powder is Ni(ClO4)2 + ANQ. I have not tested for initiation properties yet. It is supposed to be somewhat impact sensitive at
3J.

[Edited on 13-4-2023 by Hey Buddy]
UPDATE:
I have tested some properties of the Ni ANQ ClO4 salt. It burns in over flame releasing characteristic perchlorate gas products. There is no visible
colored flame as is the case with TACP or other copper perchlorates. I was unable to detonate a sample in confined Al foil heating. Flame heating in
confinement caused Al foil to rupture emitting gas. I have not tested impact or friction for these compounds because my primary interest is flame
sensitive salts.
[Edited on 13-4-2023 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
"Nitroimino tetrazole"
I was considering testing some nitroimino tetrazole salts from ANQ. I refreshed on some literature and realized there is not really any data. Shreeve
is practically the only person to work on it. Literature claims ~10.3 km/s Vd and ~48 GPa. There is no sensitivity data at all which leads me to
assume that it probably is detonating in solution or on drying and isn't able to be handled. Its salts, which are somewhat less powerful, have
sensitivity data listed, so I assume those are more accessible. Id really like to prepare this free molecule. Unless anyone's already been there, I'm
going to move forward assuming a detonation is going to occur. I believe the use of HNO2/AcOH could be handled in polypropylene to minimize frag
during detonation.
Does anyone have any experience with Nitrimino tetrazole? Thanks.
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
IIRC you can prepare nitraminotetrazole (which I think is the same thing) by nitration of ordinary 5-aminotetrazole. I think it was Shreeve et al that
published this method, but I don't have the paper with me.
I think the nitrogen rich salts (the usual suspects - ammonia, (amino)guanidines, hydrazine, aminotriazole, etc.) were reportedly somewhat more stable
than the free acid (as you might expect).
edit:
Klapötkes group has published a paper on it:
https://www.researchgate.net/publication/230161097_Nitration...
I think you can read most of that study in Stierstorfers PhD dissertation which is publicly available here:
https://core.ac.uk/download/11032575.pdf starting on page 130
There is also some work on the corresponding 1-N-oxide:
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10....
[Edited on 17-4-2023 by Microtek]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Thanks for that dissertation, I had never read that. I have been attempting to condense ANQ into 5-Natz with this scheme:
Attachment: ANQ Reactivity.pdf (5.8MB)
This file has been downloaded 254 times

I haven't had success yet but found some material with very descriptive precipitation methods, IIRC from Lieber but I cant recall. Ive had some
trouble getting precipitations in salts. I was excited at the prospect of a lead salt, which was attempted by ANQ+NaNO2+AcOH followed by lead acetate.
It appeared to precipitate an insoluble white salt but after drying, it doesnt explode and is unreactive. Im left in the dark yet, I may try to
replicate a known thermally explosive salt first like the Ca, simply to verify there is success in conversion to nitrimino tetrazole. I will synth a
supply of ANQ to return to this effort.

I also attempted to cyclize ANQ into whatever sort of triazole analogue forms from formic acid. Similar to what is found for aminoguanidine. I assume
either a nitro or nitrimino. With excess concentrated formic acid treatment there is definitely a reaction. ANQ solution changes from orange/brown to
pink and then white/cream. I attempted to first precipitate something by addition of water to the acid solution. The ANQ+formic makes a white
lava-lamp layer beneath the water which was filterable. Next I attempted to react this crude material with Ni(ClO4)2 similar to the AGu procedure for
NAP. That didnt seem to work.-- I assume perhaps I will reduce formic acid to stoichio balancedinstead of excess, and dissolve ANQ into H2O first
before addition of formic. If a nitrimino or any triazole is formed it should complex easily with metal perchlorates. It's hard to know if the track
of effort is correct until you can get it to detonate on flame, which I suppose is the point of it all.
[Edited on 20-4-2023 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Lieber worked on the route to 5-NATZ and some salts from ANQ. He has detailed procedures I will attempt to reproduce in:
Attachment: The Reaction of Nitrous Acid with Nitroaminoguanidine.pdf (435kB)
This file has been downloaded 211 times
Semenov worked with heavy metal 5-NATZ salts including lead:
Attachment: 5_NATZ_heavy_metal_derivatives_semenov2007.pdf (842kB)
This file has been downloaded 252 times
There isn't a clear indication of the explosive behavior of semenov's heavy salts. I assume they are explosive similar to heavy metal tetrazole salts.
Lieber describes the Potassium salt as exploding with purple flame. The free acid exploding with orange flame. It seems possible they could act as
primary explosive materials of themselves without introduction of oxidizer. There is discrepancy between Shreeve and Klapotke on Vd and P of the free
acid, it's believed to be somewhere between 9.3 km/s and >10.3 km/s. Known attempted primary explosive salts of 5-NATZ include Ca and Ba. Calcium
salt has some hydration forms that change behavior making it less desirable for traditional detonator form.
Next attempt is to isolate the sodium salt first and then the free acid. From a successful sodium salt, complexes for DDT, with a focus on primary
explosive DDT behavior.
Other related material includes this patent for 5-NATZ complexes.
Attachment: 5_NATZ_gas_generators_US5516377.pdf (729kB)
This file has been downloaded 227 times
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Klapötke et al published a paper on the diaminourea salt of NATz (attached) but it is just on par with RDX both in terms of performance, sensitivity
and thermal stability.
Attachment: PEER_stage2_10.1002_zaac.201000231.pdf (2.8MB)
This file has been downloaded 205 times
They have also published a paper on the hydroxylammonium salt which might be more interesting, but I can't access it just now.
[Edited on 21-4-2023 by Microtek]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
In terms of ANQ preparation, there are some things I've learned: Firstly the stirring of the NQ+Hydrazine solution can continue far beyond the 15
minute mark recommended in literature. I often continue stirring for as long as needed to entirely dissolve all NQ. Temperature also seems to not be
limited to 60 but can rise to 70 C without diminishing yield.
I have been adding 2x mol NaOH to Hydrazine sulfate to free it in water, leaving in all the sulfate in solution without separation, adding this
solution directly to heated NQ solution. There is also a literature method by Ross Phillips 1928 for ANQ using hydrazine sulfate and ammonia solution,
to which NQ is added and the heat raised to 60 C which then proceeds with the typical ANQ reaction yielding ~50%.
Attachment: ANQ PHILLIPS.pdf (398kB)
This file has been downloaded 211 times
The yield of ANQ is generally low 35%-50%.
There is a large amount of amber filtrate left over after filtering off ANQ. In the past this solution has been considered waste solution. This ANQ
filtrate can be left standing for at least a week with no precipitation of anything. It's believed the solution consists of a range of soluble
byproducts including diaminoguanidine and aminoguanidine. A CO2 gas generator was run into the filtrate which precipitated needle crystals immediately
that fell to the bottom of the jar. The filtrate jar was left standing overnight. A mass of block crystals formed on the bottom of jar. The remaining
filtrate could be poured off while the crystals attached themselves to the glass. Image of two materials on filter paper is crude ANQ and the
carbonate crystals of presumably diaminoguanidine/aminoguanidine. ANQ in its pure form is white, this crude ANQ is yellow until recrystallized from
hot water.
  
On another note, I previously attempted to cyclize ANQ with formic acid followed by attempted complexation with Ni perchlorate. The cyclization
appeared successful and the complexation appeared unsuccessful. The attempt at a Ni(ClO4)2 Triazole seemed not to react in a heated solution but only
mix. After drying this material there is certainly energetic potential for this cyclization product. The incomplete mixture of perchlorate and azole
doesnt detonate on flame test but it does entirely consume Al foil at rapid speed in combustion. Ejection of material also happens on exposure to
flame both confined and unconfined. I still think the two materials were not properly complexed due to the lack of noticeable change during reaction
and non-homogenous color of mixture after drying. It's possible that pH adjustment is necessary to initiate reaction along with heat.
[Edited on 21-4-2023 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Na 5-NATz, CHNaN6O2, 152.0474 g/mol
I have used an adaptation of Liebers K salt method to successfully precipitate Na 5-Nitriminotetrazole aka Nitramino Tetrazole. This procedure used
crude, unrecrystallized ANQ for expedience. This procedure also avoids use of acetic acid, simplifying reagents. There seems to be negligible loss of
yield from water medium in lieu of glacial acetic acid.
Solution (A)
2.38 g ANQ .02 mol
25 ml dH2O
Solution (B)
1.38 g NaNO2 .02 mol
10 ml dH2O
Solution (A) was heated to 70 C, solution (B) was added resulting in temperature drop of ~10 C.

Reaction mixture was heated back up to 70 C and held there.
The solution changed from opaque tan to clear yellow.

Once the solution became clear, stirring at 70 C was maintained for 15 minutes.
After 15 minutes of stirring, reaction vessel was placed in Na salt/ice bath to rapidly cool.
White crystallizations began to appear throughout the vessel in ~10 minutes.

Cooling was continued until 10 C.
The reaction mixture was filtered, the filtrate was saved and evaporated, revealing additional crude Na NATz.
The material can be recrystallized from water with some loss, but should be pure enough to be used crude for displacement by other salts or dropping
out the free acid later.
This procedure was repeated at .04 molar equivalent of ANQ (4.76 g).
The .02 mole (2.38 g ANQ) yielded 1.6 g.
The .04 mole yielded 4.00 g.
These yields were from material in filter not including material from filtrate evaporation.

The Na 5-NATz is a light tan powder. It burns violently with crackling over foil unconfined and deflagrates with a thud followed by crackling in light
foil confinement. There was no DDT found in flame tests.
It is clearly more violent in open burning than ANQ or ANQN.
I'm unaware of published toxicity on NATz but would assume they are all acutely-toxic, similar to azide.

This is the combined filtrate from the two runs after evaporation. The downside of using unrecrystallized ANQ is that the filtrate will hold all the
impurties which contaminate the remainder. Thats why this additional material is so yellow/orange.

[Edited on 23-4-2023 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
I tried to complex the carbonate product from the ANQ mother liquor, harvested earlier, with some nickel perchlorate. I thought if there were
carbonates of diaminoguanidine or aminoguanidine it would reveal its energy in a perchlorate complex. The resulting material had no color change from
reactant mixture to final product, and was inert and unreactive over flame, turning a yellow color and losing its green hue. This causes some
insecurity on the identity of the crystals taken from ANQ liquor after infusion of CO2. I believe DAGu should be fairly obvious from reaction over
flame. I don't know what material is dropping out of solution on CO2 introduction.

|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
I'm currently stuck on the free acid. So far I've not found a great way to precipitate anything. I have attempted modifications of Lieber's free acid
with HCl on H2O over mild heating. All goes according to plan but no obvious precipitate on cooling. There seems to be some "trace" precipitate, but
much less than literature on the procedure via potassium salt route. I'm leaving it on ice now, longer than prescribed. Trying to get something to
come out of solution. May attempt to evaporate reaction mixture for a free 5NATz and NaCl combo. Regardless, it certainly appears that freeing the
acid is successful due to change of opacity on addition of HCl. The trouble is isolation. Maybe an addition of alcohol or other solvent, nitromethane?
I don't know.
I have also attempted another try at a Pb (5-NATz)2*4H2O. Its still in limbo but appears to form a different product from earlier attempt. Hopefully
something can be found in NATz family which undergoes BTFU soon, Id rather not post too much about non-ddt materials. I'm not going to spend much time
on anything found without flame responsive DDT. The free acid is priority in this regard, thanks to it's performance and purported detonation over
heat. That much is claimed by literature, it's detonation is described as "shattering", it serves as an obvious preliminary benchmark in testing NATz
compounds. IF I can find it...
more to follow...
[Edited on 23-4-2023 by Hey Buddy]
UPDATE: Free acid attempt precipitated nothing overnight on salt ice bath. Evaporating now. Free acid has dec. at 140 C with reported detonation. Im
evaporating at 60 C.
[Edited on 23-4-2023 by Hey Buddy]
Pb salt seems to not have displaced Na in attempt at Pb NATz. The procedure tried was Pb(C2H3O2)2 dissolved in 15ml H2O @ 66 C. Na 5-NATz was mixed
with H2O and added after Pb acetate dissolved. Tan powder the same hue as sodium salt was the result. According to Semenov, who used nitrate iinstead
of acetate, there should be large prism crystals precipitating after 1 Hr of mixture. He doesnt describe application of heat when reacting. The Na
NATz dissolves in water above 70 C so I may attempt reaction again at higher temp with pre heated NATz in solution
[Edited on 23-4-2023 by Hey Buddy]
|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Hey buddy. Good work. I heard ANQ is actually white when recrystallized. I saw pictures and was shocked because it’s so deeply yellow.
What do you think has the most promise for a safe and extremely powerful energetic? I think the metal complexes rule out anything other than a primary
in most cases, but definitely not all.
Love the work you’re doing man. We really need a 9500m/s safe secondary
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by MineMan  |
Hey buddy. Good work. I heard ANQ is actually white when recrystallized. I saw pictures and was shocked because it’s so deeply yellow.
|
I don't know if the work is "good", but it is work. So far, there seems to be more learning about what isn't desirable vs finding anything desirable.
Hopefully something useful will shake out. But thank you for the compliment.
--Yes, the ANQ is white after recrystallizing. I have tried to use a dirty ANQ but it may end up being the case that contamination causes problems and
recrystallization is necessary. not sure yet. The sodium salt seems to be energetically responsive enough to indicate successful cyclization to
tetrazole from the ANQ, but its not as obvious as say a free acid high velocity detonation would be.
Quote: Originally posted by MineMan  |
What do you think has the most promise for a safe and extremely powerful energetic? I think the metal complexes rule out anything other than a primary
in most cases, but definitely not all.
|
I think ionic compounds and metal complexes are the current frontier. It seemed like it was being pushed this way by BNCP, DBX-1 etc. already, but I
think this "field" has the most gold in the mine so to speak. Industry in china has moved from things like DDNP to complexes like GTG carboyhdrazide
perchlorates for industrial blasting. I think in terms of primary explosive character control, complexes are the logical field that still needs
expansion. The misunderstanding of perchlorates and lead by the regulatory people seems to be holding it back on focus, but the halogen compounds have
probably the most to offer.
I thought as you did about metal explosive complexes being somewhat less useful other than primary explosives, but after experimenting in this area
over the past couple years, I have found that there are a much larger number of insensitive explosive complexes (less mechanically sensitive than say
PETN) found in this area. The primary explosive character of short DDT I've found is somewhat rare and harder to locate. golden eggs.
I do think, in terms of big picture, primary explosives are more needed than secondaries. But simply produced ones. The NAP has the most going for it,
I think if it were a little better explored, it may offer a flexibility on routes and expedience/power that other primaries don't have.
--Carbohydrazide and semicarbazide ligands are also of interest because they are from urea directly without need to derive guanidine first. Minimal
processing and maximum expedience of reagents/preparation is desirable for any new primary explosive in my opinion. In that way, I appreciate SADS. It
is the simplest primary explosive other than say mercury fulminate, or HMTD. The trouble in organic peroxide behavior is they are all technically
auto-decomposing all the time. Heat, moisture, radiation or pressure accelerates the auto decomp, which rules them out IMO, unless that can be
overcome. SADS on the other hand appears to have no faults other than it is slightly anemic, and it reverts to silver on radiation. I have found in my
own tests that it actually lasts a long time and is very explosively responsive to heat after long storage, without direct sun. SADS is somewhat
insensitive by primary standards and detonates in small single particles. The preparation route is short with minimal hazard. I think it is the
fastest yielding explosive. The need of silver can be expensive, but a little goes a long way in SADS. I have been testing SADS with NC binder (10%+)
and I really like what I've found. I would like to try a sun blocking material, maybe graphite or PTFE, to increase shelf life, but I think there is
potential in SADS as a pellet, with another boosting explosive after it, like ETN.
For secondaries, I think there are definitely some simple ionic secondaries to be found yet which will be powerful. Nitrates/perchlorates/periodates.
Glycine perchlorate at least for small production seems like it may be worthwhile.
Hexamine derivatives, although there is a lot of data on them already, seem to also have some more potential, and hexamine is very simple and
universal in preparation so it is a natural area to be further explored.
Guanidine and Urea also. IMO because secondary explosives have to be produced in high quantity, materials they are derived from should be very easy to
produce industrially as well as being expansive in their routes and variants etc. This is the problem of TNT as an early secondary, there is
practically only TNT, TNT mixtures and HNS in that family tree, which is limiting. Versus something like guanidine which is unknown how far it
extends. Guanidine can already produce many secondaries of different powers and sensitivities or primaries. Industrially or on the small scale, it's a
more efficient family tree. Im not so sure about NATz though, it's getting cumbersome, if its powerful it could have some logical use. it seems like
they are high temperature decomp compounds 200-400 C. I mostly just wanted to keep tack of all the tests on this thread so anyone else curious can see
what I tried.
I think we should aim for 10k+ and see whats found on the way.
[Edited on 23-4-2023 by Hey Buddy]
|
|
|
Microtek
National Hazard
   
Posts: 872
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Hey Buddy:
With regards to isolating free NATz, you could try extracting with a polar solvent - maybe ethyl acetate for starters followed by distillation of most
of the solvent and slow evaporation of the rest. If free NATz is fairly acidic, there's a decent probability it will also be fairly soluble in water.
I also suggest that you purify your reactants as much as you can. Impure reactants makes identification of the products exponentially harder because
you can't really rule anything out. If you don't have access to advanced analytical equipment (most of us amateurs don't) that uncertainty makes it
almost impossible to positively identify what you have made.
I think that metal complexes are very useful for primaries, and that organic and/or halogen ionic compounds are very promising for secondaries, but I
don't think it will be easy to find a high performing metal complex secondary. The metal tends to be dead weight since it typically doesn't add to the
energy release. Exceptions can possibly be found, but it would have to be a pretty exotic compound. There has been some Chinese papers on
metal-organic networks that claimed extremely high performance, but they turned out to be pure fantasy (probably. They were based on assumptions and
methods that were blatantly wrong).
|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Hey Buddy. Thank you for the long reply! Any more info on what you said China is using for blasting? I know US and AUS blasting companies are very
slow to change in this regard.
NiAqPerchlorate is, stunning. The copper version is actually less sensitive and more brisant. It does not ddt in microgram quantities like the nickel
salt, but it is far less sensitive. Even the nickel version. I saw a picture of it in an aluminum tube that was hit with a hammer and it did not go
off. In my mind, these two salts have stopped me from reading about research in primaries, because, they are basically ideal. Stability might be an
issue. I saw once, with the copper salt that it was very insensitive when made. But a few months later it went off when barely touched with a hammer,
just a small portion of one sample that was divided, I had never seen that before and didn’t quite believe it.
I find secondaries more interesting because, well NAQPerchlorate is basically the ideal primary, it’s quite remarkable the preparation and the raw
performance.
|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | Hey Buddy:
With regards to isolating free NATz, you could try extracting with a polar solvent - maybe ethyl acetate for starters followed by distillation of most
of the solvent and slow evaporation of the rest. If free NATz is fairly acidic, there's a decent probability it will also be fairly soluble in water.
I also suggest that you purify your reactants as much as you can. Impure reactants makes identification of the products exponentially harder because
you can't really rule anything out. If you don't have access to advanced analytical equipment (most of us amateurs don't) that uncertainty makes it
almost impossible to positively identify what you have made.
I think that metal complexes are very useful for primaries, and that organic and/or halogen ionic compounds are very promising for secondaries, but I
don't think it will be easy to find a high performing metal complex secondary. The metal tends to be dead weight since it typically doesn't add to the
energy release. Exceptions can possibly be found, but it would have to be a pretty exotic compound. There has been some Chinese papers on
metal-organic networks that claimed extremely high performance, but they turned out to be pure fantasy (probably. They were based on assumptions and
methods that were blatantly wrong). |
I remember those papers, the silver complexes that they claimed 11.3kms.
Microtek, do you think the Urazine perchlorate is still the most powerful secondary? It certainly was not too excotic in preparation. If hey buddy is
looking for the ultimate primary he should try metal complexing the Urazine perchlorate… it’s far denser than aminoguanidine as you know. I think
we talked about this but I have been having memory issues since long covid… and can’t remember a thing.
|
|
|
underground
National Hazard
   
Posts: 704
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
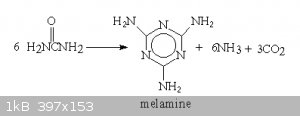
For powerfull secondary easy to make explosives to my mind it comes melamine dinitrate/diperchlorate. Melamine can be made by just heating up urea
|
|
|
dettoo456
Hazard to Others
  
Posts: 249
Registered: 12-9-2021
Member Is Offline
|
|
@underground Melamine is a pretty solid building block for EMs, sadly DNAM (it’s fully nitrated counterpart) isn’t stable enough to be used
practically. The perchlorate like you mention supposedly has a density of 1.94g/ml which is promising but conflicting sources reference it as a
hydrate or dihydrate salt, which doesn’t bode well for primaries. If you are interested, there’s a thread for this exact topic “Melamine
dinitrate”. Philou has some good observations - triaminoheptazine (melem) might be an even better EM base seeing as its N to C ratio is higher. And
density isn’t too different. If metathesis rxns can be done on the bases too, then melamine and heptazine nitrate and perchlorate salts could prove
very interesting.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by Microtek  |
regards to isolating free NATz, you could try extracting with a polar solvent - maybe ethyl acetate for starters followed by distillation of most of
the solvent and slow evaporation of the rest. If free NATz is fairly acidic, there's a decent probability it will also be fairly soluble in water.
|
I have been demoralized on the NATz project, ive been taking a leave of absence, heavy beer drinking. I did attempt evaporation which looked
promising, a clear lemon colored substrate was left in beaker. No detonation. Alas, it is a dead end.
Quote: Originally posted by Microtek  |
I also suggest that you purify your reactants as much as you can. Impure reactants makes identification of the products exponentially harder because
you can't really rule anything out.
|
It is true and in the case of NATz I suspect in the route from ANQ, contamination is compounded. It may necessitate recrys at each step. I was
attempting to avoid this for expedience but as roads run out it makes it necessary to repeat with recrys.
Quote: Originally posted by Microtek  |
I think that metal complexes are very useful for primaries, and that organic and/or halogen ionic compounds are very promising for secondaries, but I
don't think it will be easy to find a high performing metal complex secondary. The metal tends to be dead weight since it typically doesn't add to the
energy release. Exceptions can possibly be found, but it would have to be a pretty exotic compound. There has been some Chinese papers on
metal-organic networks that claimed extremely high performance, but they turned out to be pure fantasy (probably. They were based on assumptions and
methods that were blatantly wrong).
|
I agree that the metal is dead weight in contribution to density, but in my understanding, the metal provides scaffolding that permits tunability of
a complex with oxygen and nitrogen as the major players in power and hydrogen as a sensitization indicator which also helps in scaffold work.-- I
suspect the character of metal complex explosives (which are really simply ionic explosives with bridging metal ligands) is controllable in terms of
sensitivity and power, along with n-oxide addition, it seems that some very purposefully sought after characteristics could be found after the general
understanding is learned about. They are still trying to decide what to call explosive complexes in the literature, whether MOFs etc.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Sure, the major abrupt change they have made in blasting composition in china right now, from DDNP, which was very extensively used for general civil
blasting there since the 90s, is tris(carbohydrazide) metal perchlorates. Carbohydrazide aka diaminourea, is urea with two additional aminos from
hydrazine. It can be produced in a few routes other than urea also. The main two "CHZ" primary explosive complexes the chinese have used are: zinc
tris(carbohydrazide) Di(perchlorate) which is known as GTX, and the cadmium analog, which is Cd(CHZ)3(ClO4)2 known as GTG. GTG I believe is the winner
over time attrition in detonator compositions. The reason, is because China maintains a large supply of carbohydrazide for export as an industrial
oxygen scavenger in factory equipment world-wide and so the carbohydrazide complexes sort of materialized. Then out of all the complexes, the cadmium
had the most compressed late-stage DDT which meant high-impulse, and it seemed more brissant than the others.-- They also apparently used Mg, Ni and
Cu which also do purportedly work as primary explosives but in the end, Zn and Cd remained.
Quote: Originally posted by MineMan  |
NiAqPerchlorate is, stunning. The copper version is actually less sensitive and more brisant. It does not ddt in microgram quantities like the nickel
salt, but it is far less sensitive. Even the nickel version. I saw a picture of it in an aluminum tube that was hit with a hammer and it did not go
off. In my mind, these two salts have stopped me from reading about research in primaries, because, they are basically ideal.
|
I agree. I am very impressed by the Ni analogue and I think when it is successfully synthesized, it is my favorite primary explosive. I like
everyhting about its character. There are issues with it's synthesis routes that lead to probably variable complexation which gives a range of
characteristics, but even the weaker firing NAP is very powerful. When the variability is figured out, I think it will be a natural azide replacement,
assuming regulatory control allows a perchlorate. Regardless, it is definitely what I would consider ideal. I will be using it a lot more, for sure.
Quote: Originally posted by MineMan  |
I find secondaries more interesting because, well NAQPerchlorate is basically the ideal primary, it’s quite remarkable the preparation and the raw
performance.
|
This is a good point, once the primary is settled, and it doesnt take much, then the secondary is really the greater interest. There are a lot of
considerations of secondaries, IMO simplicity of secondaries in their manufacture is critical, because quantities of secondaries are so large in most
applications. Any intensive process or aspect of preparing a secondary, is compounded by the quantity needed. In this regard, I suspect that ionic
secondaries are the most promising. In traditional military munitions for instance, say aircraft bombs, the largest payloads are ammonium nitrate
based and in small cases NQ based insensitive large bombs. This is due to the complication of large payload munitions and the preparation of material
in large mass devices. Higher performance, simple materials, like ionic explosives, could entirely change this. Not that I think dropping bombs is a
good thing, I only mean to use that as an example of present limitations in its scale.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by underground  | For powerfull secondary easy to make explosives to my mind it comes melamine dinitrate/diperchlorate. Melamine can be made by just heating up urea
|
I have done some experimentation with melamine as a ligand and it makes very insensitive complex secondary explosives. I havent done any performance
tests like hitting steel or aluminum, but I really like the melamine molecule. More than melamine, theoretically, is melem, which is one higher step
in condensation above melamine, it increases nitrogen percentage and velocity/pressure stats. The highest Ive read about would be Melem n-oxide
perchlorates/nitrates which are theorized to be high velocity 10km+. I have not had the opportunity to attempt preparing melem from melamine. So i
have no experience with that.

In America, I have recently found that "Oxone" is now available as a pool chemical at the hardware store under the name of "pool Shock". Oxone is used
to oxideize molecules to N-oxide and is probably the best chance at melamine/melem n-oxide based iionic compounds. I also suspect oxone is capable of
oxidizing TMTN to RDX which is difficult.

|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by underground  | For powerfull secondary easy to make explosives to my mind it comes melamine dinitrate/diperchlorate. Melamine can be made by just heating up urea
|
It’s 7kms VOD. But it has outstanding thermal stability. I thought around 300C. But powerful, no.
|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hey Buddy  |
Sure, the major abrupt change they have made in blasting composition in china right now, from DDNP, which was very extensively used for general civil
blasting there since the 90s, is tris(carbohydrazide) metal perchlorates. Carbohydrazide aka diaminourea, is urea with two additional aminos from
hydrazine. It can be produced in a few routes other than urea also. The main two "CHZ" primary explosive complexes the chinese have used are: zinc
tris(carbohydrazide) Di(perchlorate) which is known as GTX, and the cadmium analog, which is Cd(CHZ)3(ClO4)2 known as GTG. GTG I believe is the winner
over time attrition in detonator compositions. The reason, is because China maintains a large supply of carbohydrazide for export as an industrial
oxygen scavenger in factory equipment world-wide and so the carbohydrazide complexes sort of materialized. Then out of all the complexes, the cadmium
had the most compressed late-stage DDT which meant high-impulse, and it seemed more brissant than the others.-- They also apparently used Mg, Ni and
Cu which also do purportedly work as primary explosives but in the end, Zn and Cd remained.
Quote: Originally posted by MineMan  |
NiAqPerchlorate is, stunning. The copper version is actually less sensitive and more brisant. It does not ddt in microgram quantities like the nickel
salt, but it is far less sensitive. Even the nickel version. I saw a picture of it in an aluminum tube that was hit with a hammer and it did not go
off. In my mind, these two salts have stopped me from reading about research in primaries, because, they are basically ideal.
|
I agree. I am very impressed by the Ni analogue and I think when it is successfully synthesized, it is my favorite primary explosive. I like
everyhting about its character. There are issues with it's synthesis routes that lead to probably variable complexation which gives a range of
characteristics, but even the weaker firing NAP is very powerful. When the variability is figured out, I think it will be a natural azide replacement,
assuming regulatory control allows a perchlorate. Regardless, it is definitely what I would consider ideal. I will be using it a lot more, for sure.
Quote: Originally posted by MineMan  |
I find secondaries more interesting because, well NAQPerchlorate is basically the ideal primary, it’s quite remarkable the preparation and the raw
performance.
|
This is a good point, once the primary is settled, and it doesnt take much, then the secondary is really the greater interest. There are a lot of
considerations of secondaries, IMO simplicity of secondaries in their manufacture is critical, because quantities of secondaries are so large in most
applications. Any intensive process or aspect of preparing a secondary, is compounded by the quantity needed. In this regard, I suspect that ionic
secondaries are the most promising. In traditional military munitions for instance, say aircraft bombs, the largest payloads are ammonium nitrate
based and in small cases NQ based insensitive large bombs. This is due to the complication of large payload munitions and the preparation of material
in large mass devices. Higher performance, simple materials, like ionic explosives, could entirely change this. Not that I think dropping bombs is a
good thing, I only mean to use that as an example of present limitations in its scale. |
Nickel Amino Guanidine diP is easy to prepare consistently… it strikes me as more straightforward than even ETN. There is a method. The color, the
deep red color is large crystals, the orange color is small crystals. I prefer the small orange micro crystals. It’s very consistent and takes 5
minutes for the whole process. It turns blue, then green, then black then red.
[Edited on 25-4-2023 by MineMan]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by MineMan  |
Nickel Amino Guanidine diP is easy to prepare consistently… it strikes me as more straightforward than even ETN. There is a method. The color, the
deep red color is large crystals, the orange color is small crystals. I prefer the small orange micro crystals. It’s very consistent and takes 5
minutes for the whole process. It turns blue, then green, then black then red.
[Edited on 25-4-2023 by MineMan] |
I suspect under patent conditions, it's consistent, there are complications in the correct complexation if the patent procedure is strayed from. It is
yet"unknown" to researchers and munitions manufacturers, so there is no in-depth investigation of it. Im not aware of any literature on it other than
the german patent, and maybe some very old german lab publishings. Im aware of the recent Cu paper. --If it were researched and understood in
industry, and if they could control scalability, then the performance aspect is advantageous over other primaries. --I agree that it is a very simple
procedure, and to boil something for 5 minutes is more straight forward than nitrate ester preparation. However, the preparation of AGu and Ni
perchlorate requires more steps and less common precursors than erythritol and nitric acid. --That is interesting about the color of the size of the
crystals, I hadnt noticed that yet. but it makes sense... There are definitely things that influence the complexation to make different performance
character possible. This seems to not be encountered if the procedure is followed, but exactly what is going on is probably not understood. That's not
insurmountable, but compared to lead azide, or mercury fulminate, the reaction and reagents are basically fool proof and scalable in the masses that a
primary would be industrially produced (~kg qty.) I dont doubt NAP is suitable for scale, but its just so new, (relatively). It needs some
professional development to really displace any legacy system. I like it a lot. I think it has many advantages. Its character is rare.
[Edited on 26-4-2023 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Pb (5-NATz)2*4H2O, 537.3764 g/mol
lead 5-nitroaminotetrazolate

This material detonates aggressively.

It can detonate in single grains and seems to be quite powerful, seemingly more than most common primaries.
 
Pb 5-NATz is made from Na 5-NATz in the same way as lead azide from sodium azide. I used Pb(C2H3O2)2 in this attempt.
I don't currently have any tetrazole salts to side by side in comparison. --I think this stuff is pretty powerful.
It's responsive to flame while somewhat higher temp in dec point (230 C) than say Lead Azide at 190 C, (It goes without saying, it's much more
powerful).
In this molecule, the Pb is divalent with NATz, similar to azide, but with hydration.
It is at least insensitive enough to handle for small scale testing. I haven't tested friction or impact yet, but will return to that at a later time
after comparisons with some other primaries. Non-hygroscopic. Insoluble in H2O.
The amount used here in image is around ~5 mg.
Personally, I appreciate lead as a metal in primaries. Im less concerned with Lead products than say nickel. Lead is a universally accessible heavy
metal which is lower toxicity than others, desirable attributes for general purpose heavy metal primaries.
I made this without recrystallizations throughout, just crude materials.
The preparation could likely be done wet, without drying in between ANQ->Na(NATz)->Pb(NATz)2, from ANQ forward, reducing time to stage.
I was satisfied with this material's relative preparation simplicity and high-performance.
I see 5-NATz as less tedious than a comparable tetrazole via AGu.
Looking forward to more testing on this one...
[Edited on 2-5-2023 by Hey Buddy]
UPDATE: been doing some more small detonation tests, this PbNATz is impressive. 10 mg detonations are very powerful. I dont know what velocity its
detonating at, but I think it may be interacting with the Al foil in confined detonation. Making a flash that i dont see in open, unconfined
detonation. It's really powerful. No doubt.
[Edited on 2-5-2023 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
It's now the next day, I've been testing this stuff this morning and afternoon. I have to say, this is seemingly the most powerful explosive per
volume that I've yet seen. I suspect it's more powerful than the NTz molecules but I don't really know that without direct comparisons of explosive
work. At 10 mg+ tests, this stuff is getting slightly intimidating, approaching small rifle fire, in terms of the perceived impulse. The gas output
itself is relatively low at such small scale but oh boy is it loud and powerful. I believe it is reacting with the aluminum foil in lightly confined
tests. There's no foil left other than where it is clamped in place. I may try a wax witness plate on detonation to see what kind of size the Al foil,
if any, is turned into on detonation. It is possibly vaporizing to Al2O3 or some other mixed product. Wax seems like it could possibly capture foil
particles from small tests to analyze destructive brisance.
I did rudimentary friction and impact tests. I would consider this material insensitive. I generally use PETN as a comparative benchmark and this
material is less sensitive to impact than PETN. In fact, I was unable to detonate samples on an anvil, striking with a 32 oz hammer. I was unable to
set a sample off by 32 oz hammer pressed and dragged across the material on top of anvil steel.
I dont want to talk it up too much, but really can't stress enough how impressive this material detonates. It is so loud and violent and fast. It
definitely creates luminous sparks from the Al foil on detonation when lightly confined.
It appears to be somewhat less flame responsive than I originally had thought. I have attempted some fuze ignitions in foil with simple visco and they
failed to detonate. Probably due to poor heat contact. Those tests were very crude foil/fuze tests, with small masses of the Pb NATz. Not the best
source-study of flame sensitivity but I think it needs a nice hot direct transmission for detonation. If you give it proper heat activation it really
fires. Wow.
|
|
|
| Pages:
1
2
3 |
|