| Pages:
1
2
3
4 |
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Glycine Perchlorate
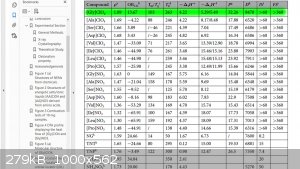
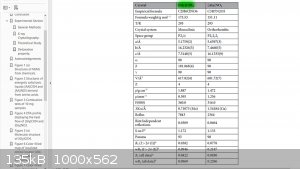
I began looking into amino acid ligands. Glycine perchlorate is reported to be:
1.89 g/cc
+13.67% (CO) OB
Vd 8470 m/s
32.26 GPa
>60 J IS
>360 N FS
mp 103 C
Td 263 C
("Insensitive Ionic bio-energetic materials derived from amino acids" Zhang, "Scientific Reports" 06 Oct 2017)
This got my attention.
If that information is accurate, this would be good.
I wanted to double displace with glycine ammonium perchlorate and HCl, it didnt happen, here's why...
The first thing I did was heat up 50ml H2O to around 90 C then added .083 mol NH4ClO4 with a matching molar of glycine. At some point during boiling,
I detected ammonia with my sniffer. The next thing I knew I was looking at a thick white paste. I put it on a hot plate, it started melting weakly
around 140 C or so. I put some under a propane torch, it seemed to melt and let off higher energy perchlorate response with the occasional flash and
blue white light.
I then repeated with .485 mol equivalents. This time I refluxed in an open beaker. I first heated the NH4ClO4 in water to around 80 C and had no
detection of ammonia. Glycine was added, the temp dropped a little then around 85 C bubbling began and the smell of ammonia was detectable. Litmus
paper was hung over the beaker and it gave an alkaline color change ~7-8 pH. Ammonia is being evolved from NH4ClO4 after glycine is added to a boiling
solution. I refluxed this, adding water to reduce foaming until I couldnt detect a positive ammonia smell over the beaker, around an hour, fifteen
minutes of boiling. Then it was poured into a few hundred ml isopropanol and a precipitate separated to the bottom of the beaker. It melts on a hot
plate much lower temp... Probably around 103 C, it has a wide melt temp shelf, I took it up to 145 C and it melted easily, no decomp.
Glycine decomp is 233 C, NH4ClO4 is 200 C. Neither materials melt.
I think this may be glycine perchlorate. It's drying right now.-- It's possible glycine perchlorate can be made by refluxing glycine and ammonium
perchlorate until ammonia is lost. If that is in fact what is happening, and if the specs on glycine perchlorate are correct, it's the easiest 8400m/s
32 GPa 103 mp material that I know of. Ive found threads on glycine nitrate. There is nothing on glycine perchlorate. It blows away most amino acid
salts in performance, the closest follow up is iso-leucine perchlorate at 7900 m/s and aspartic acid nitrate at 7500 m/s 22 GPa. BTW ASP.NO3 has an mp
of 98 C and Td of 183 C. TNT is 6800 m/s 19 GPa mp 80 Td 295.
[Edited on 19-12-2022 by Hey Buddy]
[Edited on 19-12-2022 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
  
 
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
I got some aspartic acid. Obviously I'm going to test feasibility of boiling displacement of ammonium nitrate/aspartic acid in the morning.
Perhaps guanidium nitrate ASP or Glycine... I believe glycine might condense as a cyclic.
[Edited on 19-12-2022 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Aspartic acid in NH4NO3 does not have the same effect as the perchlorate. There is no detectable liberation of ammonia and no change of litmus paper.
It definitely looks like the addition of glycine to ammonium perchlorate is displacing the ammonia. During the repeat test with glycine, I dipped a
litmus paper into the solution and it gave a slight acidic response, this would suggest free perchloric acid is in the solution.
The only thing that is unclear to me is if this is making proper glycine perchlorate. The reason is the dried product is not very reactive to flame.
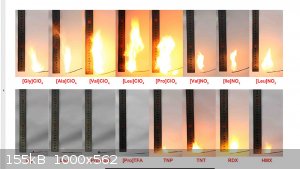
In the referenced literature there is a picture of GlyClO4 combusting with a clear 10 cm flame. When I hit this produced material with a torch it lets
of sparks and decomposes into black residue with melting at surface. There is no big flame. Perhaps it could be crystal structure? Possibly not dried
well enough?
I also wonder if the simple displacement is only attributed to glycine alone or if it is possible with other larger carbon amino acids.
I suppose a test of glycine in NH4NO3 would be good and a test of aspartic acid in NH4ClO4. That could give a better idea of what is generally going
on.
Im stumped on flame size not being the same as the referenced test. The GlyClO4 in picture is clearly more vigorous burning than controls of TNT RDX
TNP and HMX. In the tested displacement phenomenon, there is only NH4, ClO4 and Gly present. If ammonia is liberated, which it is, then there can only
be glycine and perchlorate both of which have much higher bp than water. It should be producing GlyClO4. I don't know why the reactivity appears much
lower than it should.
Here is a copy of the referenced paper on amino acid ionic explosives.
[Edited on 19-12-2022 by Hey Buddy]
Attachment: insens_bio_energetics.pdf (3.4MB)
This file has been downloaded 352 times
[Edited on 19-12-2022 by Hey Buddy]
I also realized tonight there is an Ammonium Glycinate. Glycine can act as acid or base. Seems unlikely as the favored reaction due to free emission
of NH3, could be a subtle contaminate.
[Edited on 20-12-2022 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
I found a report of glycine perchlorate embedded in a thread here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=36...
Quote: Originally posted by enthalpy  |
Now to the new stuff: Glycine perchlorate
I found absolutely no information about it. PATR 2700 only mentions the nitrate salt and the nitramine (nitroglycine). But the nitrate salt is said to
be as powerful as picric acid.
I started as for HDPC. Dissolved the glycine in a bit of destilled water and adding perchloric acid slowly. Afer addition of acid was complete there
was no trace of crystalisation. So the beaker was put in the fridge and cooled to 0°C. Still there was no single crystal. I remembered that urea
perchlorate has similar properties (in 100ml water over 900g of urea perchlorate can be dissolved). It is also very hygroscopic and crystals are very
hard to obtain. But anyway I gave GP another try. This time with success:
14,4g of perchloric acid (70%; 0,1 mole) was cooled to 5°C and stirred electrically. 7,5g of aminoacetic acid (0,1 mole) was added slowly. The slow
addition and stirring is important because there could form hot spots (you know…..perchloric acid and organics aren’t that good). The temeprature
of the mix rose a bit. After addition was complete all of the glycine dissolved. The mixture was a viscous and clear liquid. Next step was a bit
crazy. The beaker was put in a water-bath and heated to 80°C and held there for 20min. Still it was a liquid. This was crazy because boiling down a
solution of an unknown organic perchlorate isn’t harmless. The beaker was put into the fridge. Crystalisation occurred. The crystals were scraped
out of the beaker and instantly packed airtight. Tests were performed with the following results:
Hygroscopicity: GP is definitive hygroscopic. If exposed to the air it “melts”.
Solubility: GP is very soluble in water, soluble in ethyl alcohol and acetone. It . is however
insoluble in non polar solvents such as naphta.
It builds colorless crystals which melts easy without decomposition. If stored airtight there was no trace of decomposition. When crystals of GP get
in contact with flame they first melt (fast and easy) and then burn down with a hissing noise. It looks quite energetic. GP can also be detonated by a
hammer blow.
I thought about the existence of salts of GP. So I made a solution of GP in water/ethanol (1:1) and slowly added potassium carbonate. Carbon dioxide
was evolved and the solution turned milkey. I put it in a waterbath and evaporated as much water/ethanol as possible. Put in in fridge and got a white
powder. Upon ignition it melts and then flashes like potassium picrate or calcium nitronate. I don’t know whether this is a real salt of GP or
simply a mixture of glycine and potassium perchlorate (the proton in the reaction could also be from the protonated amine group). Just out of
curiosity the lead (or other heavymetal salts should be tested).
[Edited on 12-3-2008 by enthalpy] |
Based on his description his material sounds close to what Im seeing in this material.
I now strongly suspect GP can be produced by the refluxing of NH4ClO4 and Glycine.
If this is GP, then the Zhang article may not be accurate. Their GlyClO4 stats might not be correct either. Im going to have to make a new personal
rule to disregard chinese researchers because I have been served so much confusion and misdirection over the years taking their reports at any literal
interpretation. Their flame color in the photo isn't really even consistent with the appearance of flames from the many perchlorates and perchlorate
compositions that are known.
[Edited on 20-12-2022 by Hey Buddy]
[Edited on 20-12-2022 by Hey Buddy]
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Yes, it is a very unfortunate trend that papers from chinese universities can't really be taken at face value. On the other hand it means that the
work we do here, attempting to reproduce their results, has actual value beyond satisfying our own curiosity.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
There are many dissertations where math errors appear like elementary school students.
For this reason, basic research here on science madness is often more valuable. Than anything else.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
specialactivitieSK
Hazard to Self
 
Posts: 94
Registered: 21-10-2014
Member Is Offline
Mood: No Mood
|
|
How about a double reaction. Glycine hydrochloride and sodium perchlorate.
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Well, that will give you a mix of the four ions. GlyP is very, very soluble in water, but it might be possible to use alcohol solutions to precipitate
the NaCl. I still think it would be easier to just make the perchloric acid and use that. IMO, the biggest problem is the hygroscopicity, just like
with UZP. I came across a paper recently about the use of co-crystallization with nitrogen heterocycles to improve some of the prperties of energetic
salts. In particular, cyclic ureas worked well, so I'm going to try urazine and maybe ethylene urea to try to improve GlyP and also UZP. I will just
have to try with a range of molar ratios since I don't have any way to predict the hydrogen bonds that are most likely to contribute.
|
|
|
Etanol
Hazard to Others
  
Posts: 187
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hey Buddy  | I began looking into amino acid ligands. Glycine perchlorate is reported to be:
1.89 g/cc
+13.67% (CO) OB
Vd 8470 m/s
32.26 GPa
>60 J IS
>360 N FS
mp 103 C
Td 263 C
("Insensitive Ionic bio-energetic materials derived from amino acids" Zhang, "Scientific Reports" 06 Oct 2017)
[Edited on 19-12-2022 by Hey Buddy] |
How was estimadet VoD and detonation pressure?
It is very much for these compound at 1.89g/cc. May be c.a. 8000 m/s 25 GPa?
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Etanol, this is exactly what I mean. The paper uses photos of burn tests suggesting the materials burn vigorously more so than RDX control sample. The
photo line up and charts in combination are convincing enough, although they seem exaggerated. I'd assume their numbers are calculated not measured. I
dont think they faked the photos, because that would be a lot of effort and wouldnt make sense. But I'm not yet convinced that the results are
reproduceable nor accurate. My intent was to show how glycine was displacing ammonia from its perchlorate. I do think the amino acid materials need a
lot more investigation, they definitely melt, so that makes it worthwhile. I havent had time nor weather to do much experimenting lately. Id like to
test some glycine perchlorate from HCLO4 to compare and see if their burn tests are repeatable that way.
|
|
|
Etanol
Hazard to Others
  
Posts: 187
Registered: 27-2-2012
Member Is Offline
Mood: No Mood
|
|
Yes, I read this article now.
I do not dispute the photo results. Salts of chlorine acid burn very briskly really. But burning does not determine the VoD. Therefore, the
calculation method or software is interesting.
Methylinam and ethylendiamine perchlorates have a greater heat of the explosion, but generate no more than 7600 m/s at 1.68 g/cm3. Guanidine
perchlorate is only 7150 m/s at 1.67 g/cm3 because It has a lower heat of the explosion. However, with a maximum density of 1.743, its speed is
similar.
Glycine perchlorate has even less heat of the explosion than guanidine.
[Edited on 27-12-2022 by Etanol]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Sorry, didnt mean to suggest you disputed photos, I just meant that I thought they were unexpected, color and shape of burn unexpected to me compared
to other perchlorates and size. I doubted reproducibility and their calculated values. The data they determined is probably best-case in *theory. I
would prefer it if the data were accurate, because then I could stock up on glycine perchlorate.  . .
I have found that relative ratio CHO and the percent of N and organic/inorganic links can somewhat predict behavior profile of explosives, but only
generally. The theoretical high density seems realistic from molecule shape. It seems like a glycine ionic compound could be brisant. Its a small
amino acid, it would make sense if the other amino acids were more sluggish. That seems to be the general rule they also calculated. Most of their
compounds calculated were in the 6-7km/s range. The glycine perchlorate is an outlier.
I assume that displacing ammonia out of its perchlorate using glycine is giving a contaminated product. I would like to test it in detonation because
of its simplicity. Experiment with maybe a much longer reflux or alkaline/acidic reflux condition. Even if refluxed material were detonating in modest
performance, it would be nice. At the cost of glycine and the lack of other critical materials, no acid etc. if it were detonatable say in 6km/s
velocity or higher, then it would be a meltable explosive that could be mass produced inexpensively. That would be worthwhile. If it could somehow
achieve their claimed performance it would be incredible, but I doubt.
Samples made from ammonium displacement are burning nothing at all like their claimed burn profile, but they do melt and do react to heat. That would
suggest glycine perchlorate is produced but maybe it has contamination of unconverted NH4ClO4 or Ammonium Glycinate. If it can be determined how to
push reaction to GlyClO4 with that method, it would really qualify as a high yielding inexpensive OTC explosive preparation. IMO to produce it using
HClO4 is fine in small quantity, but it is a bit laborious to produce for a lot of material. So displacement is preferable. If it were possible via
displacement, you could buy glycine and make a perchlorate cell for your salt, then drop it out as ammonium cation. It would be a magnitude simpler
route to achieve 8km+ melt cast explosive, rather than say K-6/RDX. Also insensitive. Not sure what critical diameter would be but I dont see why it
would be large. If critical diameter were low, and high performance were realistic, GlyClO4 would have a lot going for it. You can find glycine
~$10-20/lb but it doesnt have to come from limited pyrotechnic stores. If the ammonium displacement method could be worked out, no acid would be
necessary, which is a considerable savings. The perchlorate can be had at the cost of NaCl and NH4 and kW/hr/$.
Doing a control run with HClO4 to see what the pure product does is the first thing I'll do and if it is different in behavior then I will know what
to work towards in displacement and if there were any hope in that route.
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Maybe co crystallization will work if for example Urazine is used along with the glycine? Would electricity help the displacement? Maybe heat is
needed. Sometimes these solutions need to be 70 plus C for the Metathesis to happen. Of course the great PHILZOU would know… perhaps he can be
summoned through the spells of old chemistry books.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Thanks to Mineman for bringing this topic to my attention,
Nice subject.
The acidity data from aminoacetic acid (glycine) and ammonia/ammonium are consistent with the observed evolution of NH3(g) upon mixing and heating of
NH4ClO4 and H2N-CH2-CO2H.
NH3 + HClO4 --> NH4(+) + ClO4(-)
H2N-CH2-CO2H --> H3N(+)-CH2-CO2(-) (the proton of the carboxilic part of the molecule can be taken by the basic amino group next by.
NH3/NH4(+) pKa is arround 9,24 vs water
pKa's of H2N-CH2-CO2H are 2,34 vs water for the -CO2H part and 9,6 for the -NH2/-NH3(+) part.
This means the basicity strenght of ammonia/ammonium and amino/aminium are in the same range...and are hungry for HClO4 but NH3 is volatile what amino
part of glycine is not... so with a moderate heating (strong heating is not advisable due to perchlorate salt of organic amine are better kept
relatively in a safe zone of temperature range).
NH3 will thus leave the system quite fast with good venting and/or vaccuum.
(NH3 is an old war gas keep that in mind for eyes, respiratory tract and nose/nostrils/lungs protection).
So NH4ClO4 is a source of HClO4 in disguise by displacement of the volatile base NH3 thanks to a less volatile equivalents strenght base.
The carboxilic acid part is effectless vs the strong acid HClO4 (at least a million times stronger in acid strenght).
The performances of glycine is of course better than the other members of the usual amino-acids perchlorates because amine perchlorates performances
are dictated by:
-Oxygen balance of the salt as CO2 (and not as CO what is informative but less interesting following me)
-Structure of the amine ... what is strongly related to the basicity and salt formation with strong acids like HOClO3 (HClO4).
-Structure of the molecule and presence of energic fuel sub-structure.
Glycine is H2N-CH2-CO2H and you may look at it as CH3-NH2 + CO2
It is the simpliest tiny one and if monoperchlorated near the perfect OB... close to CH3-NH2.HClO4...
CH3-NH2.HClO4 --> CO2 + HCl + 1/2 N2 + 2 H2O + 1/2 H2
HO2C-CH2-NH2.HClO4 --> 2 CO2 + HCl + 1/2 N2 + 2 H2O + 1/2H2
Any other structure brings more fuel but without increasing the amount of perchloric groups linked to it via extra amino groups of strong basicity,
it will no be able to burn from the in situ generated oxygen during the detonation process; so density will be lower and extra fuel uncorrectly used.
The interest of amino-acids is evident as readily available start products and OTC ones; but, of course, any good chemist would know how to design
artificial new ones of chozen structure that will surpass natural ones with regard to density, number of amine groups, basicity, energy content of the
skeleton and displaying better detonic performances.
I think that metathesis with NH4ClO4 is of value if taking time and precautions but indeed the route via HClO4 is probably more straightforward and
rapid despite the need for cooling and probable tempering by water that must be taken rid of afterwards.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by PHILOU Zrealone  |
So NH4ClO4 is a source of HClO4 in disguise by displacement of the volatile base NH3 thanks to a less volatile equivalents strenght base.
The carboxilic acid part is effectless vs the strong acid HClO4 (at least a million times stronger in acid strenght).
|
Interesting. So if you wanted to try this simple displacement on other molecules would you look for target molecules with NH2 see-sawing against a
weak acid?
also, the glycine perchlorate made from simple displacement doesnt burn as intensely as an RDX sample. The reference line up burn test of GlyClO4 has
a ~5 cm reaction flame, much larger than their rdx control sample. Is there an obvious reason for this? (The GlyClO4 from displacement was made by
boiling for an hour. And another time was made by boiling for only ~20min, neither burn with large flame, both melt.)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Hey Buddy  | Quote: Originally posted by PHILOU Zrealone  |
So NH4ClO4 is a source of HClO4 in disguise by displacement of the volatile base NH3 thanks to a less volatile equivalents strenght base.
The carboxilic acid part is effectless vs the strong acid HClO4 (at least a million times stronger in acid strenght).
|
Interesting. So if you wanted to try this simple displacement on other molecules would you look for target molecules with NH2 see-sawing against a
weak acid?
also, the glycine perchlorate made from simple displacement doesnt burn as intensely as an RDX sample. The reference line up burn test of GlyClO4 has
a ~5 cm reaction flame, much larger than their rdx control sample. Is there an obvious reason for this? (The GlyClO4 from displacement was made by
boiling for an hour. And another time was made by boiling for only ~20min, neither burn with large flame, both melt.) |
What do you mean by "see-sawing against a weak acid?"?
I would rather play with HClO4 dilluted (10-72%) and a pure or dissolved base or putative base containing a NH2 group.
Indeed if NH4ClO4 was under hand I would probably test it by under vaccuum fusion and evolution / trapping of NH3...
While some perchlorate are quite unsoluble and easily recovered cristallized by precipitation; other are hell hygroscopic and would require
considerable heating, vaccuum or dehydration (via dehydrator) in close container and protection from air moisture to avoid remoisturization in a short
time.
This is the case of N2H5ClO4 (very sensitive (shock, friction, flame, heat and impact) and powerfull but also very hygroscopic compound).
I could dry my hydrazine nitrate into an hermetic box over an ambiance radiator (40°C) holding also hydrazine perchlorate... the HP turned a full
liquid leaving my HN very dry solid.
Such hard to solidify and to dry compounds are also hard to detonate because of the last bit of contained water of crystalization (often cought from
the air or from other chemicals)... during exposure to heat, they usually melt into their crystallization water, evaporate and decompose or burn at
the same time and this tempers a lot the heat of decomposition, burning, explosion or detonation /power.
It needs super drying... but this increases a lot sensitivity to friction, shock, flame or heat.
I have played with H2N-CN (cyanamide what is if you take a close look at it a dehydrated form of urea...)
H2N-CO-NH2 <--> H2N-C#N + H2O
So cyanamide is a hydrolitic source of urea (and then further of ammonium carbonate) into the soil as fertilizer, it is also a good fuel because less
water trapped into the structure and more multiple bond as energy stored source... Cyanamide should logicaly display a better combustion heat than
urea.
It must be slightly more acidic than urea (this last is neutral vs water but basic vs HClO4) so cyanamide must stil be able to form salts with the
amino group against strong acid like HClO4... forming cyanamide perchlorate (CAP). Cyanamide is a weak acid despite the amino group because of the
viccinal electron withdrawing cyano group but HClO4 is very strong acid and it will thus turn the weak acid into a weak base in its own perspective
and respect.
H2N-CN when trimerized could be seen as melamine (-N=C(-NH2)-)3 (since cyanamide is dehydrated form of urea melamine is also a trimeric form of urea
tripplely dehydrated  )... isn't it fabulous how chemistry is logical )... isn't it fabulous how chemistry is logical  and consistent with itself... and consistent with itself...
Cyanamide heat of combustion must be higher than melamine (due to the stabilization induced by the (poly-hetero-aromatic ring
formation/polymerization/cyclic trimerization what implies loss of energy content for stabilization by aromaticity).
Melamine perchlorate and diperchlorate (cannot fix a third perchloric acid molecule into a stable melamine tri-salt)... those are already quite good
performer vs urea perchlorate (overoxygenated).
N#C-NH2.HClO4 should beat performances of melamine diperchlorate... but it is too rich at oxygen so OB is positive (a problem melamine diperchlorate
circumvent by lacking a HClO4 molecule)... Overoxygenated (positive OB) means a loss of energy for full detonic power of CAP.
The extra oxygen become a unused dead weight... it lacks fuel.
This problem is shared by all positive OB explosives.
There must be an optimal mix of melamine diperchlorate and cyanamide perchlorate that display a perfect OB and thus maximum power output.
Cyanamide does form a dimer with itself called di-cyanamide what is in fact cyanoguanidine...
H2N-C#N + H2N-C#N --> H2N-C(=NH)-NH-C#N
A probable intermediary on the way to melamine by trimerization and cyclization.
Cyanoguanidine is a very interesting powerful molecule... it has more energy content than guanidine because one H had become a C#N...
The guanidine part allows good quality as a base and ease of salt formation vs oxidizer carrying acids...
It was tested as an experiment with a friend about 30 years ago and it detonated strongly... it was made from HClO4 and chemically pure bought
cyanoguanidine.
My friend had access to chemically pure chemicals thanks to his father water analysis society. Good memories  . .
CGP has a good OB (close to 0) but is stil slightly lacking oxygen for a perfect OB.
H2N-C(=NH)-NH-C#N.HClO4 --> 2 N2(g) + HCl(g) + 2 H2O + 2 CO(g)
Would require one mole equivalent of O2 for perfect OB and maximum energy output...
2 CO + O2 --> 2 CO2
RDX is cristallizing relatively dry and burn well... it seems logical that hygroscopic perchlorate of amines catch water from the air (or unperfectly
dried product) and are then tempered a lot in their explosive behaviour or burn rate vs a truly dried sample.
The large flame diameter from glycine perchlorate vs RDX is probably linked to its explosive power (sensitivity to flame and initial burning rate in
the open or slighly self confided... read probable faster B2D(2D) transition (burning to deflagration to detonation transition) mass from self
burning)... it expels itself in the air and arround while burning more vigourously.
That is what I think explains your observations.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Sorry, this term is hillbilly-pre-realiztion of what defines an amino acid.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Thank you for your clarification.
Amino acids are easy because existing relatively-OTC and cheaper...but limited in structure / design.
Artificial amino acids can be done via a Strecker synthesis from a keton or aldehyd and an aminium cyanide...
R-C(=O)-R' + R"-NH2 + H-C#N (R"-NH3-C#N) --> (R-)(R'-)C(-C#N)(-NH-R")
Where R, R' and/or R" can be alkyle, aromatic or H.
The later addition product upon acidic or basic hydrolysis provides an amino acid... but is this important ? (Because a cyanide / nitrile group is
more interesting energetically speaking.
This opens the way to un-natural polyamino and polycarboxylic acids (or polycyanido compounds) allowing for denser poly perchlorate salts on a
molecular level (better OB/power)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Of course playing with aminium cyanide must be done with due caution owing to the inherent composition ... soluble cyanide anion and potential free
cyanhydric acid upon exposure to the slightest weak acids like H2CO3 (CO2 from air or breath and air moisture) or aminium (NH4(+) or R-NH3(+) are weak
acids)...
Thus only for skilled chemist that understand the danger of such procedures.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Glycine is a good example of desired amino-acid.
Retro-Strecker is pointing into the direction of formaldehyde CH2=O (CH2(-OH)2).
CH2=O + NH4-C#N --> H2N-CH2-C#N (perchlorate of glycine nitrile must be very potent)+ H2O
-H(+)-> H2N-CH2-C(=O)-NH2 (diperchlorate of this glycin-amide must be very strong and performing, also good density)
-+H2O-> H2N-CH2-C(=O)-O-NH4 (ammonium glycinate)
From this observation it would be an excellent idea to test for Strecker synthesis onto dicarbonyl compounds like glyoxal
O=CH-CH=O + 2 NH4CN --> N#C-CH(-NH2)-CH(-NH2)-C#N
This last as a diperchlorate will be outstanding because as a dimeric kind of Glycine nitrile perchlorate, it will be denser; about the same
sensitivity/stability...but with a slightly better OB thus more energy output...
2 (H2N-)(N#C-)CH2.HClO4 -theorical dimerization-> H2 + (H2N-)(N#C-)CH-CH(-C#N)(-NH2).2 HClO4 (bis-glycine nitrile diperchlorate)
This should result into a very good hyperperforming energetic material with very good detonic parameters...just like the bis-glycine amide
tetraperchlorate or the bis-glycine diperchlorate.
This is only a tiny examplative sample of my ideas onto the subject of synthetic artificial amino-acids...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
glycine sulfate and sodium perchlorate maybe? ammonium perchlorate is a pain on its own- for safety we should maybe try to produce glycine chlorate as
homemade perchlorate can oftenly contain some chlorate
as for getting it out of solution, i would imagine somehow getting the GlyClO4 into maybe- acetone, then adding in an excess of polar solvent, or a
mixture of solvents that shifts the polarity and crashes out the salt
https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/mar...
this would claim acetone isnt miscible with anything, methanol seems to go into hexane and heptane, this is very easy to get from lighterfluid for
zippo, distill out of gasoline, around 70*C fraction, or sold under names such as "washingbenzine" in europe
now, a sodium salt was mentioned- if thats possible, should an ammonium salt also not be possible to be formed? ammonium picrate was used a lot.
forming a salt could maybe make it easier to pull out of solution
maybe good ol' DCM can help? the hygroscopicity is a major drawback, but the added difficulty could hopefully hint at it being very powerful
ammonium permanganate can also be made- and may be possible to combine in similar fashion, its a fair bit more unstable than ammonium perchlorate, so
it could potentially be some kind of primary
perchlorate salts are very attractive especially if one can make them from chlorate by melting, half an hour at 300+*C didnt seem to do much for
sodium chlorate. sodium perchlorate can be extracted using acetone- coming to think of it, glycine sulfate could maybe be combined with sodium
perchlorate in acetone? and then simply shift the polarity, or let the acetone evaporate- as it usually does very well due to its high vapor pressure,
let this occur at a very slight vacuum to supposedly keep moisture out and you would have a very practical route
EDIT: glycine chlorate is a real thing and its a primary! albeit, in the form of a coppersalt.
https://www.youtube.com/watch?v=K0X0qZF6DeM
[Edited on 11-1-2023 by Antiswat]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Glycine + Hydrazine + Ni + NH4ClO4 makes a blue nickel crystal. Very energetic. Not sure if it's a suitable primary explosive, drying it out
completely, but when wet state the crystals fire gas jets strong enough to extinguish flame.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Hey Buddy  | | Glycine + Hydrazine + Ni + NH4ClO4 makes a blue nickel crystal. Very energetic. Not sure if it's a suitable primary explosive, drying it out
completely, but when wet state the crystals fire gas jets strong enough to extinguish flame. |
It can be too many things...
H2N-CH2-CO2H + H2N-CH2-CO2H --> cyclo(-NH-CH2-C(=O)-)2 (diketopiperazine) + 2H2O
For info: Most amino acid do form diketopiperazine while heated/decomposed.
Such molecules are interesting precursor for nitramide formation by concentrated mixed acids nitration with cooling and without water.
Dinitro-N,N'-Diketopiperazine from glycine must be quite interesting...
See cyclo(-N(-NO2)-CH2-C(=O)-)2
or
C4H4N4O6 --> 4 CO + 2H2O + 2 N2 + heat
It lacks 2O2 for complete combustion and full energy output during detonation.
It must be close to keto-RDX for sensitivity to shock/heat and for hydrolysis.
And close to keto-RDX and RDX for density...
What can it be?
H2N-NH2 + H2N-CH2-CO2H --> H2N-NH-CH2-CO2H + NH3(g)
HO2C-CH2-NH2+ H2N-NH-CH2-CO2H --> (-NH-CH2-CO2H)2 + NH3(g)
(-NH-CH2-CO2H)2 -ox-> HO2C-CH2-N=N-CH2-CO2H + H2O
Into the mix you have NH3, N2H4, Ni(ClO4)2 and the above glycine related molecules (amino-glycine, glycine, hydrazino-diglycine, diazodiglycine) that
can make a complexating mix of variable and unknown composition... making colorful complexes...
More likely it can be Ni(NH3)x(N2H4)y(ClO4)2
with x=0,1,2,3,4,5,6
and y=1,2,3
and with x+2*y =6
Of course due caution must be applied with such due to the legendary dangerous Ni(N2H4)3(ClO4)2 reputed to detonate into watery solution from
friction/shock between spoon and glass recipient while hand swirling..
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 429
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
--Philou, that is excellent info on the diketopiperazine. I think a nitramide variation is interesting but the diketopiperazine should in itself make
its own metal complexes shouldnt it? I think tetrazole lover in the past prepared piperazine extracted from OTC dewormer. IIRC in a comment he stated
that piperazine perchlorate complexes were some of his preferred initiation compounds. I can attempt a controlled decomp. I have aspartic acid and
glycine on hand.
--If it assists in making it more clear, the preparation I used to come up with the blue crystal was approximately:
Molar equivalents of perchlorate and glycine heated in minimum water, nickel wire was added in excess with stirring, soln turned bright green after an
hour or so. Alcoholic hydrazine was added in molar equivalence. Solution was heated/stirred at around 30C overnight. Solution was royal blue next
morning and was then gently air dried in an open container until crystals remained. They are still in drying but I may try to get a picture of them
tonight and if they seem dry I may try to get a cautious idea of the sensitivity. I have read of some impractically-high-sensitivity Ni/N2H4/ClO4's so
I will be careful in handling. It is a small sample and I will be prepared for detonation. I was thinking that complexing the perchlorate with the
glycine and nickel before the hydrazine may be the safest way to experiment and it seems like it went through perhaps at least one intermediate
complex changing from green to blue. Im not really sure though.
edit:
It is not flame or impact sensitive like a primary explosive. It burns like an angry version of a nitramine where the mass shoots around on foil. It
produces a good amount of smoke. Not sure on residue because it burns holes through foil. A burning test can only give little idea of profile but in
terms of things I've burned, it seems to have the most violence in burning, not like a ddt substance but more like ANQ or K-6. But with very little
flame. And a bit of smoke. Mostly shooting out jets and smoke. Something about it just seems angry though, hard to explain. It seemed like only small
spots burned reacting to flame but they burn with a lot of gas pressure and noise so it makes you think it's going to detonate, but it just burns. It
seems to be holding water too. It doesnt seem to be totally dry despite drying for ~2 days on low heat. Sensitivity to hammer impact by hand seems
fairly low, maybe a little more impact sensitive than ETN. Im not in search of this kind of material right now but it is interesting. The simplicity
of Gly/ClO4 is the most intriguing. Maybe I should try nickel with hydrazine and add to GlyClO4 instead... 2,5-Diketopiperazine seems worth a test in
complexation. I assume it can be prepared from heating glycine to under the decomp temp of 230 C.
edit 2: also seems like there was a red spot or two on crystals, not sure if this is a contaminant or a different oxidation state. I burned one of the
red crystals and it behaved the same as the blue.
[Edited on 13-1-2023 by Hey Buddy]
 
[Edited on 13-1-2023 by Hey Buddy]
|
|
|
| Pages:
1
2
3
4 |