SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Synthetic sea salt
A study I did on producing synthetic sea salt. This was mostly a mathematics test.
| Code: | = Synthetic sea salt
= Seawater composition in parts per thousand (mass fraction)
NA Water 964.8 18.0 g/mol
17 Chloride 19.3 35.5 g/mol
11 Sodium 10.8 23.0 g/mol
NA Sulfate 2.7 96.1 g/mol
12 Magnesium 1.3 24.3 g/mol
20 Calcium 0.4 40.1 g/mol
19 Potassium 0.4 39.1 g/mol
NA Bicarbonate 0.15 61.0 g/mol
35 Bromide 0.07
NA Borate 0.03
38 Strontium 0.01
9 Fluoride 0.001
3 Lithium 0.00017
= Seawater composition (moles)
NA Water 53.6
17 Chloride 0.544
11 Sodium 0.470
NA Sulfate 0.028
12 Magnesium 0.053
20 Calcium 0.01
19 Potassium 0.01
NA Bicarbonate 0.0024
11 Sodium 0.470
12 Magnesium 0.053
20 Calcium 0.01
19 Potassium 0.01
17 Chloride 0.544
NA Bicarbonate 0.0024
NA Sulfate 0.028
one possible salt combination, 1 mole total:
0.94 mol NaCl
0.075 mol MgCl₂
0.056 mol MgSO₄
0.03 mol CaCl₂
0.02 mol KCl
direct conversion from g/mol to g:
58.5 g NaCl
7.15 g MgCl₂
6.74 g MgSO₄
3.33 g CaCl₂
1.49 g KCl
10 grams total:
7.58 g NaCl
0.92 g MgCl₂
0.87 g MgSO₄
0.43 g CaCl₂
0.19 g KCl |
I wonder which salts sea salt actually recrystallizes as. It's very interesting to me that table salt comprises only 75% of it.
[Edited on 2/10/2022 by SnailsAttack]
[Edited on 2/10/2022 by SnailsAttack]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
They crystalize sea salt in making table salt from seawater.
The mother liquor is called bittern, and has the bromine and iodine in it and lots of other stuff I don't recall.
If you google bittern (and avoid all the bird related definitions) you can probably find some analyses of what bittern has in it.
It probably varies a bit, as sea water varies a bit in salt content in some areas due to local minerals and degrees of concentration.
I've got some numbers for seawater from different places somewhere around here and will add to this post If I find them.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Here are some pages from a chemical engineering encyclopedia* on seawater processing which you may find interesting.

Edit: *Ulmann's Encyclopedia of industrial Chemistry
[Edited on 2/12/2022 by Plunkett]
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
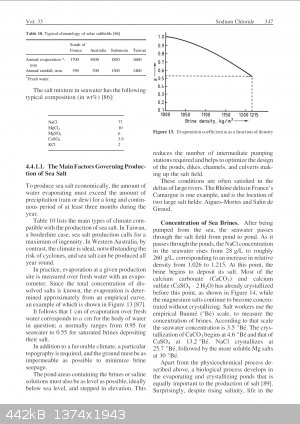
Edit: Replaced photo with better quality screenshot
[Edited on 2/12/2022 by Plunkett]
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
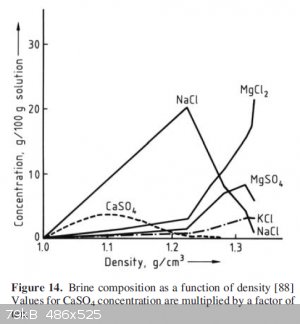
[88] Central Research Institute, Japan Monopoly Corporation, Figures for Salt Production. Tokyo 1954.
Edit: Added reference and replaced graph with better quality screenshot
[Edited on 2/12/2022 by Plunkett]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Was your scanner on mushrooms when it took that last picture?
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
I had digital access to the encyclopedia through my work, but I could not print or email myself pages so I took a photos of the computer monitor. It
is not the best, but I also never expected to be sharing the photos.
[Edited on 2/12/2022 by Plunkett]
|
|
|
Texium
Administrator
       
Posts: 4579
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Useful info to be sure, but please learn how to take a screenshot on your computer… It is also good practice to state the title of the source rather
than just saying “a chemical engineering encyclopedia.” I’d like to seek it out myself now, because I’m quite interested in seeing what they
have to say on the page you didn’t post regarding recovery from the Great Salt Lake.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
After looking at some of the other photos I took I believe it is Ulmann's Encyclopedia of Industrial Chemistry. I cannot find a PDF of the volume
that discusses the Great Salt Lake (I think it was from the one on magnesium or bromine), but I found a PDF with the other pages I posted and replaced
the photos with better quality screenshots. I am sorry for the poor quality screen shots. I took the photos for my personal reference when I had the
idea to isolate bromine and magnesium from seawater. Since I do not live near the Great Salt Lake that section was not of interest of me. Here is
one last table that compares the composition of different saline waters and it includes the Great Salt Lake as well as the PDF for the volume on
sodium chloride
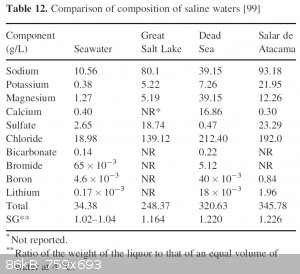
[99] H. Aral, B.D. Hill, G.J. Sparrow: "Value Adding to Salts Recovered from Saline Waters" in Disposed Basins in the Murray-Darling Basin -
Appendix 2: Production of Salts from Brines and Bitterns, Murray-Darling Basin Comission, Canberra 2004 (ISBN 1921038268)
Attachment: ullmann_sodium_chloride.pdf (942kB)
This file has been downloaded 531 times
[Edited on 2/12/2022 by Plunkett]
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Plunkett  | Here are some pages from a chemical engineering encyclopedia* on seawater processing which you may find interesting.
Edit: *Ulmann's Encyclopedia of industrial Chemistry |
awesome, thank you. i've been looking for documentation on separating sea salts by recrystallization for ages!
I really wished I lived near some saltwater lol. Lake Ontario hasn't got nothin in it
|
|
|