Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Stereochemistry of the ibogaloid catharanthine
I got interest in catharanthine, since the non aromatic pi bond should allow for some interesting chemistry and unlike the more well-known ibogaloids,
the indole ring isn't substituted yet.
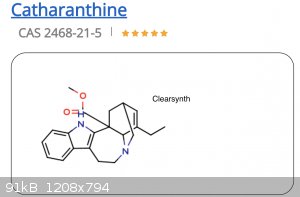
But when research it a bit, I found several inconsistencies in its stereochemistry. This is how Cayman chemicals shows it:
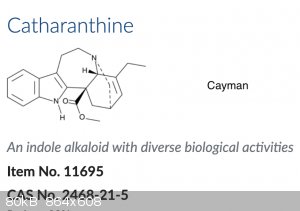
But this is how Clearsynth shows it:
The one by shown by Cayman is much more consistent with other ibogloids. So I'm wondering if anyone has some insight as to which one is correct?
Ot maybe I just not understand stereochemistry, lol.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
The first doesn't show stereochemistry, only the lines that are darker (broader) show a group is pointing towards the viewer, dashed lines point away
from the viewer
[Edited on 12-10-2021 by Tsjerk]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | The first doesn't show stereochemistry, only the lines that are darker (broader) show a group is pointing towards the viewer, dashed lines point away
from the viewer
[Edited on 12-10-2021 by Tsjerk] |
I didn't know this until now.now I know.
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Ah got it. Thanks. I guess what confused me is that in clearsynth picture the methyl bridge from the basic nitrogen to the cyclohexene ring is drawn
as if it is in the front, not in the back.
But then again, I've also never seen a hydrogen atom with four bonds to it before lol.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Monoamine  | | Ah got it. Thanks. I guess what confused me is that in clearsynth picture the methyl bridge from the basic nitrogen to the cyclohexene ring is drawn
as if it is in the front, not in the back. |
Yeah, the bridge is drawn in the front, but also if you compare
the two structures, you can see that the whole molecule is flipped relative to the Cayman structure. So in the Clearsynth structure you’re looking
at the molecule from the “back,” relative to the way Cayman shows it, thus the bridge is shown in the front, and the carboxylic acid would be
pointing back had they shown the stereochemistry there.
If you have a model kit, try building a 3D model of the molecule and rotating it around to look at it from different perspectives. You should be able
to see that both versions are accurate depictions.
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
Thank you for opening this can of worms!
There seems to be confusion over the stereochemistry on wikipedia too. The page on catharanthine shows the cage behind (with indole to the left). Ibogaine and related alkaloids have the cage in front. But on the Vinka alkaloids page, the cage of catharanthine is shown in front.
I think catharanthine is interesting as a possible precursor to ibogamine, provided it shares the same stereochemistry, which I think it does. Since catharanthine is already a precursor to vinblastine for
cancer treatment, it should enjoy commercial supply that ibogamine lacks. The best price I could find after a quick search was $210/g, but it is also prominently mentioned as an alkaloid in Madagascar Periwinkle (Catharanthus roseus). Does anyone have better leads on this compound?
|
|
|
Jenks
Hazard to Others
  
Posts: 163
Registered: 1-12-2019
Member Is Offline
|
|
After more investigation, I've found that (+)-catharanthine does indeed have the opposite stereochemistry of the alkaloids derived from iboga, which
is disappointing. I also learned that, even if the stereochemistry had been suitable, it would not be straight forward to convert it into coronaridine
or ibogamine because hydrogenation would tend to come from above the structure shown, forcing the ethyl group down, not up as shown in coronaridine
here:
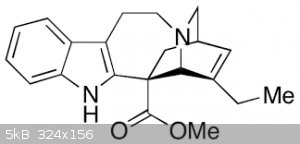 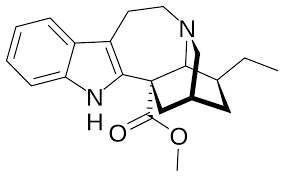
Removing the ester first might help, except that the transition state essential to the decarboxylation is too strained to proceed, as it would for
coronaridine, due to the double bond within the ring. So the double bond has to be moved first:
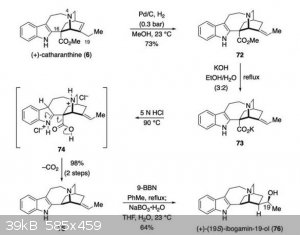
The bottom line, though, is that catharanthine is a dead end for access to iboga alkaloids because of its stereochemistry.
The sequence is from Scheme 11 of Strategies and Tactics in Organic Synthesis edited by Michael Harmata, found on Google Books.
[Edited on 11-12-2021 by Jenks]
|
|
|