Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
I want to suspend Strontium Aluminate phosphor particles in liquid...
I had an idea for a cool lava style lamp, but I'm stuck on a crucial detail to make it work...
I wanted to have phospherescent strontium aluminate circulating in a jar of liquid. It'd have a small vertical tube entirely submerged, one end close
to the bottom of the jar and open in both ends, with a small heating element attached to it, so that the heat would cause a "chimney draft". This
draft would pull up liquid from the bottom of the jar, and expel it through the top of the tube. Along with the liquid, I want it to pull strontium
aluminate powder. Then I'd have a UV LED to illuminate the stream as it travels inside the tube, so that when it goes up and out of the tube, it's
glowing brightly.
I think the effect would be quite cool, basically an eerie glowing underwater fountain.
The thing is, strontium aluminate has a pretty high density (3.56 g/cm3) and sinks quite literally like a rock in water, despite being
pretty fine grained(sort of like the finest beach sand, but not flour fine). This means there's no way it's going to be carried along with a slow flow
of water. I could probably grind it finer, but I think this may have an adverse effect on the phosphorescence, plus I would prefer to not muddy up the
liquid more than necessary since clearer ought to look better.
I thought about dissolving things in the water to make it denser, but I can't think of anything(non-exotic) that would make such a dense solution so
as to approach the 3.56 density. Plus I'm not sure what might react with the strontium aluminate and ruin it. The powder wasn't super cheap and a bit
of a hassle to find, so i'd rather not accidentally destroy it. I understand it's somewhere in the alumina/ceramics category of materials so I guess
it's not very reactive, but still. I guess NaOH could make a pretty dense solution, but I'm also pretty sure that would destroy it. Plus that sort of
strength would be a real hazard with a glass jar...
So far I tried making basically a syrup with sugar and some regular table salt. That helped a tiny bit, but not much. Thought the viscosity change
with sugar might help but I'm not really convinced... I also tried adding some sodium silicate, thinking about how it's used as a deflocculant in clay
at least. It did to some extent suspend some of the finest particles, but then it started precipitating silica everywhere lol.
I don't have a lot more ideas. Anyone?
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
This sounds a really cool project. Calcium bromide/chloride solutions can have densities up to 1.8 g/mL, and up to 2.3 g/mL if you add zinc bromide to
the mix. What if instead of using convection currents to move the strontium aluminate you used an airlift pump? Lastly, I have no idea how well this
would work, but what about mixing the strontium aluminate with a gel like calicum alginate to make the phosphor more neutrally buoyant?
Bromide brines: https://tetratec.com/products-and-services/completion-fluids...
DIY Airlift pump for sand: https://www.youtube.com/watch?v=RSbUTX0qyzs
Making calcium alginate gel: https://youtu.be/wYyqZWWU9GU?t=423
[Edited on 9/10/2021 by Plunkett]
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
I'm skeptical whether 2 g/ml would be enough. Plus it sounds like a bitch and a half to aqcuire bromide brine lol
The airlift pump would certainly work, the "sandfall" I suppose is exactly the same as what I'm doing. The inevitable noise and bulk of it is a bit of
a turn off though. Since I'm picturing the effect to be pretty eerie I think it's desirable to have as little "chaos" as possible going on. Though I
suppose there's no reason one would have to use air, the same concept should work with a jet of water I suppose? Just relying on the venturi effect. I
think I want to avoid sucking the phosphor into a pump as it is apparently hard and abrasive almost like alumina...
Hmm. I wonder if I could make the alginate. I have some "Irish Moss" used for beer brewing, and I think it's basically the same thing algin is derived
from. I wonder if it's as simple as to steep the moss in NaOH?
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
looking at this
https://en.wikipedia.org/wiki/Heavy_liquid#List_of_common_he...
suggest you will struggle to find anything dense enough without being horribly toxic
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Out of the ones listed there, saturated sodium polytungstate seems like the only reasonable option.
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Yeaah... I did consider some tungstate, I gathered they're typically the go to solution for heavy... solutions.
But I mean, say I have 500 ml of water and I wanna increase the sg to 3 or so... That probably means a kg of tungsten lol. Last time I checked it
wasn't exactly cheap, not to mention I have no clue where to get that sort of quantity. I guess I have access to a whole bunch of worn out tungsten
carbide tooling, but... Nah. Clearly density adjustment to the liquid isn't the way to go here.
I think I did manage to make some sodium- and calcium alginate, from kelp no less. Haven't tested it properly yet, because filtering and
purifying/concentrating it is just about the slimiest and slowest thing I've ever done lol. But it's getting there...
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Did you consider using something like dyed perspex powder as a phosphor?
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Have you considered silicone oil? Silicone oil is less dense than water (~0.8 g/mL), but it can be hundreds of times more viscous than water which
should slow the settling rate. Assuming Stokes' settling, at room temperature strontium aluminate will fall about a hundred times slower in 100 cSt
silicone oil than it would in pure water. If that is too fast or too slow, silicone oils come in a variety of viscosities and assuming they are all
miscible you could mix them to get the exact properties you want. The first result on Amazon shows 4 oz of silicone oil for $6.71 which would be $27
for your 500 mL. A bit pricey, but there may be cheaper sources. Mineral oil might work too. If the oil does work, I think you could get some cool
effects with the phosphor falling through an oil/water boundary.
[Edited on 9/15/2021 by Plunkett]
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
Glycerin may be a better option than silicone oil. It is denser than water (1.26 g/mL), about a thousand times more viscous than water (950 cP),
miscible with water so you can easily adjust the viscosity, and it is relatively cheap. Glycerin is hygroscopic and it seems that a few % water
significantly lowers its viscosity, so that is something to be aware of if the fountain will be open to the air. The report below details the
properties of glycerin/water mixtures including density, viscosity, and the equilibrium % water in glycerin at different relative humidities.
Attachment: Physical_properties_of_glycerine_and_its_solutions-2.pdf (7.3MB)
This file has been downloaded 725 times
[Edited on 9/15/2021 by Plunkett]
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
I'm just about ready to see what the alginate route might offer. No clue how that's gonna turn out, or if my wild guessing chemistry worked out and
resulted in sodium alginate at all lol.
The viscosity route is definitely worth trying. I guess that wouldn't be compatible with my "heat convection pump" though. Even if the powder settles
slower in a highly viscous fluid, I guess that's counteracted equally by how much slower the fluid would flow under a certain force, and provide no
"lift" benefit? I think I have a peristaltic pump salvaged from an inkjet printer laying around though, that should do well. I'd expect more of a
"water fall" than a fountain though, but that'd be pretty cool as well.
Though as far as viscous fluid goes, it seems most accessible to use plain old sucrose syrup.
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
One last thought. If you use glycerin or another liquid with a refractive index similar to glass, you could make a venturi shaped like a Bunsen
burner with the chimney made out of glass tubing. You could hide the venturi in the base of the fountain, and you would not see the glass tubing
because of the similar refractive indices. This way you can get more of a fountain effect than just an opaque tube spitting out phosphor at the top
of the fountain.
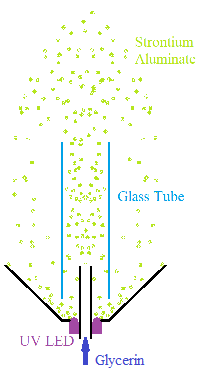
[Edited on 9/16/2021 by Plunkett]
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Cool idea! I've dabbled in glasswork, so that opens up interesting possibilities...
I managed a satisfactory solution to make the particles more bouyant, and it was dead simple too. I mixed the phosphor with normal clear coat spray
paint, acetone based. I also mixed in some sodium bicarbonate, though I'm not sure that was necessary. I then sucked this mixture up into a syringe,
and then forcefully injected it all into hot(70-80°C maybe) water.
Acetone based paint will immediately coagulate when mixed with water, since the acetone/various volatile solvents escape into to water and forces the
paint out of solution.
The water being above the boiling point of acetone will also quite violently disperse/flash boil the mix, giving a large surface area.
The bicarbonate also decomposes rapidly at this temperature, releasing co2.
The result looked like fluffy snow, and half of it ended up being so buoyant that I had to "knead" it a bit to make it sink again. It had no
noticeable negative effect on the phosphorescense either. Great success!
|
|
|
Plunkett
Hazard to Self
 
Posts: 96
Registered: 16-4-2017
Location: The Richest Hill on Earth
Member Is Offline
Mood: No Mood
|
|
That's great to hear. I did read that strontium aluminate hydrolyzes in water destroying its phosphorescence. Hopefully the paint coated the
strontium aluminate particles well enough to prevent this. Do give an update with the final product.
|
|
|