mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
thermoelectric cooling bath
https://youtu.be/0xY06PT5JDE
This video shows some peltier device that cools on one side and heats on the other. I was thinking of seating the heat side with attached heat sink in
some container with cold running water to absorb the heat, while the cold side is exposed to the water or suitable solvent. This would be nice in an
outdoor lab or for reactions that require long cooling. Has anyone else seen these things? They're pretty neat
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
https://www.sciencemadness.org/whisper/viewthread.php?tid=10...
I later got another two similar fan/heatsink assemblies so my final configuration is
two modules, each consisting of 2x (TEC1-12715 plus heatsink with fan)
(4x TEC, 4x heatsink, 4x fan total)
OK for my 10/14 distillation kit but almost useless with a 24/29 kit.
I've been away from my lab for over two years so made little progress with this scheme,
but I found that during cold weather I could distil ethanol at a reasonable rate using (24/29) condenser water recirculated through a re-purposed
domestic central heating radiator.
A quick test using the radiator plus my quad-TEC cooler seemed to be viable but I've not tested that setup enough to give a conclusion.
[Edited on 4-7-2021 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
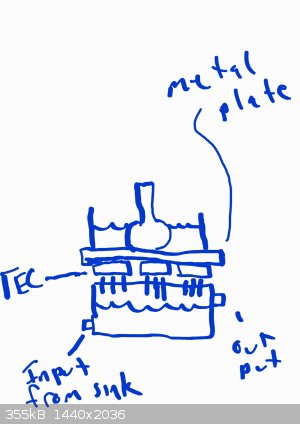
Very interesting! I was thinking to use a small tupperware that holds the water for the heat sinks, with an input at the bottom and output at the top.
With a 3x3 setup of the peltiers, that should give me plenty of room to cool down a significant amount of IPA for cooling reactions. Electroboom and
the thought emporium both have videos of making cloud chambers, and they use metal plates to hold the cold from the devices. They both reach around
-25c which is way colder than what I'm after, so an appreciable loss with the setup they use is fine by me
[Edited on 5-7-2021 by mr_bovinejony]
|
|
|
Twospoons
International Hazard
    
Posts: 1326
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
They're also terribly inefficient.
To move 1W of heat typically requires 3 to 4 W of electrical input, all of which comes out the hot side.
They're mainly used for spot cooling, not for bulk heat removal.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Rather than heatsink fins dipping in running water you could consider the cpu cooler water blocks, e.g.

much less likely to have corrosion and/or a mess.
Maintaining a low temperature bath should be practicable,
if there is sufficient thermal insulation.
Cooling a thermal runaway reaction is a different problem.
P.S. do not try glueing the TEC modules to anything,
- differential thermal expansion DOES break an epoxy resin (Araldite rapid) bond
- the TEC modules should only be operated with compressive force applied
@Twospoons - operating TEC1-127XX at 12 Vdc or more does give a very low COP,
(0.25 to 0.33 in your example)
COP >1 can be achieved at lower voltage/current,
but you need a lot of TECs to get useful heat pumping power = $$n
[Edited on 5-7-2021 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
Oh hell yeah those would be perfect! In the video he used silicon around the TECs which seems like a good idea to keep them from shifting. I'm not
sure how well thermal paste would keep them in place
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Thermal paste will keep them in place long enough for you to
APPLY THE NECESSARY CLAMPING FORCE.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
Clamping with what? Maybe some cheap wood clamps on all 4 corners?
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Anything that can maintain a compressive force ;
wood with clamps should be ok.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
If I'n not mistaken conventional compressor type refrigeration is several times more efficient than those solid state devices.
Probably be better off finding a water cooler to salvage the cooling element out of.
They're about 100 Watts but ought to be the equivalent of a few hundred watts of thermoelectric coolers.
But a couple of blocks of 'blue ice' in water in an insulated container is much simpler and can handle a much higher heat flux too.
Attachment: blue ice.webp (16kB)
This file has been downloaded 297 times
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
I have some of those blue ice things, they melt too quick and recently the ambient temp in my lab has been high enough to where an ice bath only lasts
for maybe 30 minutes. I'll look into the water cooler elements and see what I can find
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Here in Malaysia the ambient temperature is often 35C,
for an ice bath/reservoir I've been using a food box made of expanded polystyrene / styrofoam,
it makes ice last a lot longer.
e.g. https://shopee.com.my/product/85211645/4569424030?smtt=0.166...
P.S. I use the lid, leaving only a slot at one end for the water tubes and pump power cable.
Even with good insulation for the bottom and sides, ambient air melts ice from above.
[Edited on 6-7-2021 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by SWIM  |
But a couple of blocks of 'blue ice' in water in an insulated container is much simpler and can handle a much higher heat flux too.
|
Unfortunate phrase.
https://en.wikipedia.org/wiki/Blue_ice_(aviation)
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
I'd ditch the thermoelectrics for chemistry applications for many reasons:
1. The latent heat of fusion of water is huge at 334 J/g which gives tremendous cooling power. Even just 2kg of ice per hour is 185W of cooling,
equivalent to a modern domestic freezer operating continuously, or almost 1.5kW (!) worth of thermoelectric coolers.
2. If you have a freezer, ice is free and can be stockpiled. The rate of cooling is limited only by the reaction setup and not your power supply.
3. A Carnot cycle domestic freezer is around 4x more electrically efficient than a thermoelectric cooler; it will be much cheaper electrically to cool
with ice.
4. By the time you're done buying the junctions, their cooling solution, and the beefy DC supply to go with it, you'll have easily spent more than a
cheap chest freezer. You can find freezers on Amazon for less than $150, although pretty much every household already has one.
5. Peltier junctions don't move heat well. They are good at cooling relatively small items to a low static temperature but their throughput for
cooling work is low. There is a reason why they remain unpopular for refrigeration applications. They also rapidly lose efficiency when working
against high thermal gradients.
6. If you already need a circulating loop to cool the thermoelectrics, you might as well cut out the middle man and just use an ice bath with a pump
directly on the reaction. At least this way your loop only needs to handle maybe a couple hundred watts of cooling rather than a few thousand from the
thermoelectrics.
I have cooled reactors up to 50 liters with a lot of success even with reactions requiring 5°C or so using just 40lb bags of ice from the gas station
and a fountain pump. Peltiers are fancy but there is a reason they are not popular.
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
Well I already have the peltiers laying around and only need to build the rest, but before I waste time on it I'll try some different containers for
the ice. I've been using tupperware which is crap and I'm sick of buying bags of ice every other day. From the sound of it I'd be better off trying to
insulate and cool my whole lab rather than waste time on making this thing
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Gee, I always wondered what they put in those blue ice packs.
Now that's creative recycling!
Next time I need some maybe I'll just try making my own
|
|
|