| Pages:
1
2 |
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
My selenium compounds
Hi. When I was newly registered I made a mistake and now the information on selenium compounds is scattered, I decided to continue the synthesis of
selenium compounds and plus I will gather my articles here because If anyone is interested years later find it easy.
• Cu, Ni and Mn selenites + photo
• Scandium selenite and new manganese selenite + photos
• Cobalt selenite + photos
• Cadmium polychalcogenides? + Photo /sulfur and selenium
• Selenides easy preparation method
• Mercury selenite
________________________________________________________________________
•Also i made indium selenite, its white, but its just for my selenites colection. Scandium salt is also white, but ot is a very rare compound and i
dont care about colour.
 
•This is a barium selenide. It has very beautiful colour, i have much more in ampoule, but not here.

•I made small amount of red and black selenium, they aren't for reactions, because i always use granules. Red one i made from hydrolysis of green
polysenenium cation. I dissolved granules in hot concentrated H2SO4. next i heat some red selenium and made black. Should be heated but should not be
melted.

•This is cadmium polyselenide, just a fact it changed colour and get darker. Above is a link to compare.

•And boring Pb and Ni Selenides.

This is part of the selenium compounds which I found this time.
[Edited on 23-3-2021 by vano]
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
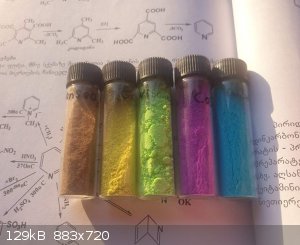
Just nice photo of Selenites.
|
|
|
Metallophile
Hazard to Self
 
Posts: 89
Registered: 23-3-2018
Member Is Offline
Mood: No Mood
|
|
Very nice! I always click on your posts, to see what new colors you've come up with.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Thanks!
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I wonder how l;ong your red selenium will remain red. I made an ampoule of red selenium myself, but now it almost lost its color and is dark grey. It
is as if the saturation of the color slowly is turned down, like colors on an old-fashioned color TV could be turned down, until you just had black
and white (grey levels).
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
When I made it, it had a very nice red color, it gradually became darker. After some period when it loses its red color I will write here.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Divalent Mercury selenite and probably samarium selenite. I used samarium chloride and sodium selenate, when I added the required amount of sodium
selenate it did not precipitated, so I added Na2SeO3 solution until it precipitate, does anyone have any idea what type of samarium selenite it might
be. I have seen mixed rare earth selenites ( for example (NdxPr1−x(HSeO3)(SeO3)⋅2H2O)), but I do not know how it is in the case of sodium.

|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Mercury(II) selenite isn't white? It clearly has a somewhat yellow color in your vial. I have a few mercury(II) salts (Hg(CH3COO)2, HgCl2, Hg(SCN)2)
and these are purely white. Mercury(II) anion is colorless. But of course, some combinations of colorless ions can lead to colored compounds, so the
yellow color may be its real color and not due to some impurity.
I also wonder how your red selenium is looking now. Is it still red? I made some half a year ago or so, but now it is almost purely grey.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Yes it has yellowish colour, i have too salts which you mentioned and i agree with you all of them are white. My mercury compounds are of good
quality. Look at the photo on this link it has a really yellowish color.
https://onyxmet.com/index.php?route=product/product&prod...
My selenium was dark gray. A few weeks ago I made dioxide from selenium granules, I did not need that powder, so I dissolved it in nitric acid with
the granules
[Edited on 8-7-2021 by vano]
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I made this crystal from mercury selenite, I melted it. I noticed that the mercury was produced, also the smell of selenium, not selenium dioxide.
Could it be selenide? Or a mixture of selenite and selenide? I then heated more and got a dark opaque, hard crystal.

|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
This is the final product

|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Quote: Originally posted by vano  | Divalent Mercury selenite and probably samarium selenite. I used samarium chloride and sodium selenate, when I added the required amount of sodium
selenate it did not precipitated, so I added Na2SeO3 solution until it precipitate, does anyone have any idea what type of samarium selenite it might
be. I have seen mixed rare earth selenites ( for example (NdxPr1−x(HSeO3)(SeO3)⋅2H2O)), but I do not know how it is in the case of sodium.
|
Also this samarium selenite in vial is only 1.62 gram. It is very light.
[Edited on 31-7-2021 by vano]
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Also it can absorb UV light very very well.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Hello! I made chromium selenite Cr2(SeO3)3 • ?H2O and cobalt selenate hexahydrate CoSeO4 • 6H2O


[Edited on 10-4-2022 by vano]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Oh, that cobalt selenate! I much appreciate your efforts to make selenium compounds, Vano. I stay away from them myself because of their toxicity.
Regarding the rare earths, I can only advise you to check a good book on RE compounds, because they oftentimes are more complicated than you would
expect. Remember the violuric acid compounds, which may include other anions, as in the attachment posted here by diachrynic.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Quote: Originally posted by Bezaleel  | Oh, that cobalt selenate! I much appreciate your efforts to make selenium compounds, Vano. I stay away from them myself because of their toxicity.
Regarding the rare earths, I can only advise you to check a good book on RE compounds, because they oftentimes are more complicated than you would
expect. Remember the violuric acid compounds, which may include other anions, as in the attachment posted here by diachrynic. |
thanks! yes i know what you mean about RE. i have some RE metals, maybe i will make some selenium salts from this metals. now i'm thinking to make
sodium fluoroselenite. i found old book in laboratory. in this book there are good selenium compounds. also it's very easy to make.
SeO2 + NaF = NaSeO3F (at 250C)
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Hi. I made cobalt selenite again and also anhydrous selenite.



|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Gorgeous!!!
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Love the colors!
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Thanks!
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Today I made selenium tetrabromide from Amorphous selenium and bromine. Then I sublimated the compound.
 
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
it gets more dark. i made insoluble complex salt Cs2[SeBr6] from it.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Wow, the colours!
Is the difference in colour compared to your first synthesis due to different lighting conditions when you took the photos, or is the result of your
second synthesis really much deeper in colour that the first?
Does it have the same colour as your SeBr4?
If the SeBr4 turns lighter, it probably turns from its beta into its alpha form (Wiki).
[Edited on 29-4-2022 by Bezaleel]
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Hi Bezaleel!
1) I think it cause because of light. but also I think in my sodium selenite solution there was some carbonate impurity. I used to make sodium
selenite from selenium. but now I have one jar of pure reagent.
also, cobalt selenite precipitation is a little bit hard than Cu and Ni selenites. I dissolved sodium selenite and cobalt chloride hydrates in hot
water. then I mixed the solution but nothing precipitated, then I poured the mixture into another beaker and there was cold water. then it
precipitated instantly. From my experience, you cant make the compound with a high yield just dissolve compounds in hot or cold water and mix them.
2)Yes they have similar colors. when it's dry I'll show you a photo.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Selenium iodide Se2I2.


[Edited on 30-4-2022 by vano]
|
|
|
| Pages:
1
2 |