aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Iodination of Paracetamol
Recently, I extracted paracetamol for use in random projects I wanted to try. One reaction that I had read about for the iodination of phenolic rings,
mono-substituted and poly-substituted, using H2O2 and I2 in water. Many of the compounds listed that they tried were similar, including
para-aminophenol (PAP). Their 1:0.5 ratio of H2O2 : I2 gave roughly 49% yield of the mono-substituted PAP and roughly 30% for the di-substituted PAP.
And the reaction was carried out at Room Temperature, or a quick heating to 50C to get the reaction started.
My question here is how to tell that I got the right compound without NMR or fancier stuff? I have DCM and ways to filter and even a chromatography
column. Their paper calls for a Sodium Thiosulfate mix to quench the byproducts and Iodine water, and then for a subsequent DCM wash. But before I
carried out the DCM procedure, I decided to do a spot test by dissolving my crudely filtered product (I didn't have Thiosulfate so I thought a simple
filtration would do) in DCM. This was disappointing to say the least. It just stayed in the water layer and did not move, but didn't dissolve in the
water either!
In reflection, am I possibly mistaking the reactivity/activity of paracetamol as opposed to PAP? Also, I tried heating at a constant 50C for a quarter
of the literature stated time (24hr reaction time), and got same result. Bunch of red precipitate from water solution, dried and filtered with vacuum,
and then spot tested by adding DCM and precipitate. Added water to see if that had any affect of the dissolution of my precipitate.
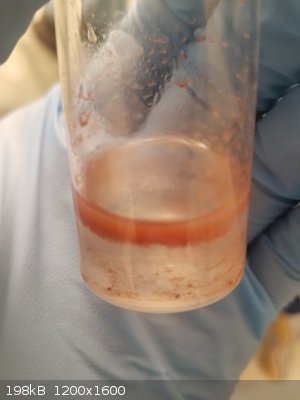
There are a few attached photos:
-Reaction mixture photo showing the ugly precipitate that is definitely hydrophobic
-Spot test with DCM
-Drying 4-acetamido-2-iodo-phenol (or 4-acetamido-2,5-diiodo-phenol)
EDIT: forgot to attach relevant literature
Efficient and selective iodination of phenols promoted by iodine and hydrogen peroxide in water - Gallo et al.
[Edited on 3-10-2021 by aab18011]
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Update, going to try an acid base cleaning. I used chemicalize db to lookup pH stats, and it seems like its soluble in basic solution. So I went ahead
and mixed a NaOH solution and dumped the dry powder in. Dissolved fairly quickly forming a dark brown liquid.
I also did a lookup on a similar analogue with known characteristics.
4-Amino-2-Chloro-phenol on Sigma says that it is "White to brown powder or crystals"
ChemicalBook.com (4-amino-2,6-dichlorophenol) says "Beige or slightly brown to pale reddish
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
reactofurnace
Hazard to Self
 
Posts: 76
Registered: 17-7-2015
Member Is Offline
Mood: Volatile
|
|
Is there melting point data? You may want to try that.
You may want to do recrystallization on the brown ppt.
|
|
|
medchemist
Harmless

Posts: 15
Registered: 10-7-2015
Member Is Offline
Mood: No Mood
|
|
Try and react away any excess iodine, that's what the thiosulfate was used for in the original procedure, sodium metabisulfite will also work for
this. any left over iodine will dissolve into the DCM with your product and complicate purification.
You could theoretically try an acid-base extraction to remove the iodine. After ridding any water soluble things, mainly the peroxide, add 1 M sodium
hydroxide to your DCM solution and extract your product into the aqueous layer as the phenolate salt and react with iodine to make iodide. You can
reacidify the mixture and extract the product into clean DCM.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Expecting to get a reasonable result, while "fudging" on multiple variables.... Is not reasonable.
Get some thiosulfate, if it is called for.
Moreover, you are traversing poorly explored territory.
Clean Paracetmol, is kind of illusive. The product is available everywhere, but seldom as a clean uncontaminated reagent.
Why not first run your experiment on phenol? Phenol is easy to make.
Interesting products. The 2,6-Di-iodo-phenol, can be refluxed with Cu and Sodium Methoxide, to yield Syringol... 2,6-Dimethoxy-phenol. Also
known as pyrogallol Dimethyl ether.
Of particular interest, because it can then be etherized via Allyl Chloride, and subjected to the Claison re-arrangement, then re-etherized, to yield
the essential oil Elemicin.
I like your project. It looks interesting. But, without the right reagents, and access to better testing equipment, it's going to be difficult to
navigate.
So.... Tell us more.
Also of note.... Were you to form the Methyl Ether of Tylenol.
That might be interesting.
But keep in mind, when you hydrolyse that Acetamide, your substrate may become "A horse of a different color". To varying degrees, Anilines are
poisonous.
Oh, yeah. The industrial production of the important anti-tubercular precursor, 3,4,5-Trimethoxy-benzaldehyde, proceeds via the bromination of
P-Hydroxy-Toluene.
As easy as bingo-bango-bongo. The P-Hydroxy-Toluene sucks up 4 Equivalents of Br2. The product is hydrolysed, etherized, and yer done. Basically.
THANK YOU FOR THE PAPER! Nice!
[Edited on 11-3-2021 by zed]
[Edited on 11-3-2021 by zed]
[Edited on 11-3-2021 by zed]
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Ah, you guys were definitely right. The original red precipitate was filled with leftover iodine, and I ended up decided to scrap it. However, I had a
bit left that I dissolved into NaOH solution and it got rid of iodine coloring, and it left a nice light brown precipitate upon HCl addition
(dropwise, 12M). I dried it and it became an even nicer light brown. As soon as the drying is 100%, I will try DCM test. Note: My P-acetamol is
definitely contaminated.
In the meantime I also ordered some Thiosulfate so I can reattempt.
As for zed's comments (thanks btw, lots of great info!), I am going to put some of my more simple reagents to use in this reaction. I'm going to try a
boat load of phenol derivatives... but makes me kinda sad to see 4 years of phenolic compounds I made go into this project. But hey, who doesn't like
testing their own hand-made reagents?
Also zed, I think I am going to use that Cu and MeO- reaction on the Di-iodo compounds I plan to make this month. I have a boat load of anhydrous MeOH
left, so this is basically going to be 'Ether' Month for me. Curious as to how many I can make before the month is over.
Reactofurnace: I must say there is scarce info on melting points of some of the iodinated compounds like this... or maybe this is just an
un-interesting compound in the world of pharma and etc.
   
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, there is a lot of iodo-vanillin posting, somewhere in the archives.
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
It seems most compounds on this list have EWGs at the 4 position, and compounds with EDGs at the 4 position perform poorly. Where do you see para
amino phenol in the paper? The one with 49% yeilds use phenol as the substrate, not PAP.
Also, paracetamol would be more sterically hindered than PAP, so thats one issue. Also, 2g(table 2) was obtained in 0% yeild, Possibly because there
are 2 EDG para to each other on the ring, just like paracetamol, but also because directing effects.
I dont really have history of being blindly accurate in my predictions, though, so take it with a grain of salt.
[Edited on 11-3-2021 by chemist1243]
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Update: Failure Again?
Okay, so I have a week to run this and do a quick extraction. I took the reaction mixture and loaded it into a separatory funnel, with ~50mL DCM. I
shook for quite some time, and then let it evaporate out slowly. All that came out was a very small quantity of amber-colored oily (but solid) dots at
the bottom of the beaker. They are so tiny and need to be inspected so closely that it pains my eye. The pictures are attached, but only one picture
shows the "product" as its so tiny. The other two photos are just to give you an idea of how small they really are.
The absorbance of 2-Iodophenol on is roughly 240 nm, so in an attempt to see if that translated to the ability to fluoresce, I flashed a UV light on
the reaction mixture, the dcm extract, and the end "product", and got nothing.
uv-spec source data
Other than that I'm at a loss of what happened. My only guess is that I need ethyl acetate, which is the alternative suggested by the paper. Is it
possible my DCM is not clean? Is it possible the di-iodo and mono-iodo phenols need an ether to help extract them? Should I have salted the reaction
mixture?
In the meantime, I only have hexane, acetone, and some di-ethyl ether on the way. However, I do not own ethanol to make ethyl acetate. I do have
glacial acetic acid.
I have also picked up some TLC plates, so I can try and test the reaction mixture in the next run. I'm going to use the recommended Hexane/DCM mix and
see if I can detect the products. I have already found some interesting stains to use. I have a FeCl3/HCl test for phenol, and I noticed that it is
really hard to get a positive with bulky side groups such as chlorine (except to say p-chlorophenol where the chlorine is totally opposite the
hydroxyl), and thus if the test is negative, then there are indeed bulky groups on the ring. My starting phenol gives a positive result 3/5 times, so
it's not as reliable. If anyone has any suggestions for a stain on this one.
Until I know anymore, my current assumption is that my reagents sucked, or my procedure sucked, or my extraction sucked, or I left my reaction mixture
to sit too long.
EDIT: I forgot to mention that this was done with Phenol, which I hope didn't confuse you when reading.
  
[Edited on 3-26-2021 by aab18011]
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
I found your chemical problem interesting since the dibromo derivative of paracetamol is well known. The attached paper may explain why you are having
trouble with your iodination of paracetamol. I guess you did not do a simple search of "iodination of N-(4-hydroxyphenyl)acetamide" before embarking
upon your experiments. Btw, much chemistry of paracetamol is referenced under its proper chemical name of "N-(4-hydroxyphenyl)acetamide."
AvB
Sorry about the misspelling of paracetamol in the filename
Attachment: Reaction of Peracetamol w Iodine banti2017.pdf (2MB)
This file has been downloaded 402 times
[Edited on 27-3-2021 by AvBaeyer]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
On the note of the paracetamol being contaminated I know from experience that most extracted Paracetamol seems to turn brown within a week even if
product is thought to be dry. Im guessing that leftover water may cause decomposition by hydrolysis or something so solvent extraction of the tablets
rather than water extraction may help
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Currently going to put this on hold until the Ethyl Acetate comes in. I also am going to design a few workups to try for this, I have a feeling I am
doing something wrong here.
Thank you AvBaeyer, that paper makes 100% sense. I think the problem exactly is that acetyl group, which behaves like acetone would with iodine, but
the ring is pretty bulky and instead acts as a donor for stabilization of the I8. My thought for a workaround for that would be deacetylation followed
by diodination, followed by acetylation. Definitely not practical by any means, but just for interest. Might try it out on the large scale to see if
it's doable.
Chemist1234 - Another idea is that I can also protect the amine in order to make the phenol more favorable. Do you think that the phenol would still
be able to iodinate if maybe I turned the amide into a salt?. Maybe the amide will become an EWG, and thus I can try and iodinate again. But so far
just ideas.
Draculic acid69 - I did solvent extraction of my paracetamol and it has yet to brown. It is a beautiful white powder. There is definitely some water
still in there, but I think it's very low.
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. I'm not sure. Seems like the paper's focus is on Paracetamol, perhaps interfering with the synthesis of Thyroid hormones. It surmises an
Iodine complex... Of not-ring-iodated Paracetamol.
Not what I would have expected, but Iodine marches to " the beat of a different drummer".
Thank you.
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Okay, so I got the Ethyl Acetate and did some work on the paracetamol. It is soluble only in Ethyl Acetate and anything homologous. I checked the
"products" and less than 1% was the iodinated compound. It worked, but so crudely. So it's safe to say this reaction does not work and requires a
different approach. I'm thinking of a pre-iodination before the acetylation. I am going to try and prepare p-aminophenol and then iodinate, then
acetylate with acetic anhydride. I'm assuming the amino group is less strong than the amino group, and so we'd still arrive at
4-amino-2,6-diiodophenol. If instead, it happens to react on the other side and result in 4-amino-3,5-diiodophenol, then I will have to try another
method, possibly iodination of phenol, then careful nitration and reduction. And then finally acetylation.
But my professor did note that nitration on a assymetrical iodinated ring can be easier than the symmetrical di-iodo compound to nitrate. He said I
may degrade the iodine before the nitration actually happens. But he said that he could find some less-known reactions to help avoid this. I have an
idea to use Zn and HCl or ZnCl2 and HCl, but not sure if the Cl in the HCl will replace the iodine.
Also as for phenol in general, the product is actually quite pure and only requires chromatography to separate the di-iodo and the mono-iodo. I was
also thinking of using it as a precursor for an iodine-based azo dye.
Updates to come...
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
|