| Pages:
1
2 |
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Beta-Alanine amino acid copper salt
After reading that most of the common amino acids form complexes with many transition metals I wanted to make some to see the colors. And the first is
is an almost perfect blue!
There is not a lot of basic info about these reactions readily available online. Beta-Alanine powder (eBay) and copper carbonate (pottery supply) was
used to make a copper salt.

I assumed the following reaction for the stoichiometry - is this correct?
CuCO3 + 2C4H7NO4 = Cu(C4H6NO4)2 + CO2 + H2O
- 21.5g alanine powder was dissolved in 120g water. It dissolved very easily, solubility as reported on Wikipedia seems incorrect or maybe my food
grade alanine is a hydrate.
- 10.1g copper carbonate was slowly added to a warm solution while stirring. A gentle reaction was observed, with bubbles being generated.
- The solution was stirred hot for some one hour.
- After an hour the solution was dark blue and there see to be no more solid carbonate left. It was then gravity filtered.
- The filtrate was a lovely deep blue liquid, and there was a very small amount of remainder, I assume unreacted carbonate.
- It was left to cool overnight, the next day at room temp there was no crystals.
- The solution was boiled down to under 50ml, still no crystals. It was then placed in an evaporating dish on a steam bath.

- After MANY hours on the steam bath the weight was constant, some solid was visible, but it was not losing weight any more. It just stayed wet. At
this point the contents of the dish was approximately 38g.

- Scratch all into a big beaker and add 150ml methanol. Boil and stir for 30 minutes. Let the ppt settle, decant, add methanol again and boil and
stir. Note the decanted supernatant methanol was purple in color.
- Vacuum filter and rinse with methanol in filter. The remainder was blue damp crystalline solid.

- Dry on steam bath until weight constant.
- 19.8g of lovely blue dry product recovered. This is a yield of about 75%.

The color was a nice surprise, the purest blue of the copper compounds I had made. It is very soluble in water.
Cobalt is in progress and I also want to try nickel.
[Edited on 17-2-2021 by Lion850]
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Worth noting: this is beta alanine you used, not alanine. In other words, the amine is attached the beta carbon rather than the alpha carbon,
like it would be in “regular” alanine, so your thread title is a bit misleading.
The equation that you stated looks fine to me other than the copper carbonate not being written as basic copper carbonate. Since basic copper
carbonate also contains some hydroxyl groups your ratios will be a bit off if you go with CuCO3 as an approximation. It’s probably not
enough to make a difference, but I would be interested to see you try it again with a more ideal ratio to see if it’s still the same color. An
excess of alanine could make the color appear more blue than it should be, by giving the copper more amines to coordinate with.
The structure of this complex is probably rather, well, complex. The dark blue color is from the amine groups coordinating to the copper ions, in the
same way that the dark blue ammine complexes form with ammonia. So the copper ions are balancing the charge of the carboxylates while also being
coordinated with the amines.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Texium thanks for the comments! Title now edited. I know the alpha and beta have something to do with left and right handedness (beyond my
understanding); does it have any effect on the appearance of subsequent compounds?
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Alpha means that substituent (in your case NH2 group) is bonded through the first carbon next to COOH. Beta means that substituent is bonded through
the second carbon next to COOH. Similary with gamma, delta, epsilon etc.
Lefthanded isomers have L in their name (like L-fructose, L-alanine, L-serine etc.)
Righthanded isomers have D in their name (like D-glucose, D-mannose etc.)
Anyway, very nice looking compound.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks Bedlasky! In practise does it make a difference to the chemistry and physical appearance of compounds?
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Of course.
https://en.wikipedia.org/wiki/Chirality_(chemistry)
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Nice work, thanks for sharing. Chirality makes quite a difference. For example thalidomide. One isomer(R) is used during toxicosis, but second one(S)
is teratogen like mercury and PCBs.
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Chirality only effects chemical properties with respect to other chiral molecules, such as biological receptors. Thus it can affect smell, taste, and
drug effects, but it will not change the physical appearance or the general chemical properties at all.
Beta alanine is not chiral, because the amine group is on the terminal carbon, which can rotate freely. Alpha amino acids (except glycine) are all
chiral because the amine can either be on the “front” or “back” of the molecule, and those molecules would be non-superimposable mirror
images, no matter how you twist them around. As Bedlasky alluded to, (alpha) alanine would have two stereoisomers, indicated as L-alanine and
D-alanine. Since beta alanine is not chiral, it has no such designations.
[Edited on 2-17-2021 by Texium (zts16)]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
I'm not sure if chirality is the right term, but that type of isomerism can actually effect reactions with non-chiral reagents. For example, some
cis-diols will form ketals while their trans counterparts will not (can't remember where I read this but if I find the ref I'll link it).
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by njl  | | I'm not sure if chirality is the right term, but that type of isomerism can actually effect reactions with non-chiral reagents. For example, some
cis-diols will form ketals while their trans counterparts will not (can't remember where I read this but if I find the ref I'll link it).
|
That's a more complex topic. Any carbon atom with four unique substituents is considered a
stereocenter or chiral center because switching the positions of any two groups connected to it will result in a non-superimposable
structure. If a molecule only has one stereocenter, such as alanine, then it is automatically considered chiral, and inversion of it will result in
its enantiomer, which would have the same physical and chemical properties, unless they are in a chiral environment. If a molecule has more
than one stereocenter, such as your diols, then there are multiple possibilities. Let's look at 2,3-butanediol.
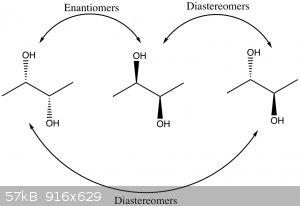
As you can see, there are three different stereoisomers. Two are a chiral pair (a pair of enantiomers) and would have identical properties in
a non-chiral environment. This is because BOTH of the stereocenters are inverted. If you only change one of the stereocenters, you end up with the
third stereoisomer. It is a special case known as a meso compound because it is not chiral (doesn't have an enantiomer). A stereoisomer that
is not an enantiomer is called a diastereomer. Diastereomers do differ in their chemical properties, sometimes very significantly. So the
meso compound is considered to be a diastereomer of each of the other two isomers.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Gents thanks for the explanations.
I need to confess....I used the wrong formula for Alanine in my original post and in my stoichiometry  I used the formula for Aspartic acid (C4H7NO4) instead of Alanine (C3H7NO2). I think the correct formula for the
reaction is: I used the formula for Aspartic acid (C4H7NO4) instead of Alanine (C3H7NO2). I think the correct formula for the
reaction is:
CuCO3 + 2C3H7NO2 = Cu(C3H6NO2)2 + CO2 + H2O
This means there were excess alanine in the solution, assuming it it not a hydrate. I'm not sure what happened to this excess; after the first
filtering stage there was a very small remainder but I assumed that was unreacted carbonate. When the product dried on the steam bath there was no
sign of 2 separate salts: usually if it is a mix one will fall out during steam bath drying before the other and often shows up as a different colored
ring in the evaporating dish.
The excess may also have mostly ended up in the purple filtrate when filtering off the methanol. I wished now I boiled this filtrate down to see what
was in it but unfortunately it was discarded.
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
You wouldn’t have seen two separate salts because what you likely have is the copper salt of beta alanine with additional beta alanine (that isn’t
deprotonated) coordinated to each copper ion. It wouldn’t be a hydrate because the alanine is a stronger ligand and would displace the water
molecules.
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Here is some literature to copper(II)-beta alanine complexes.
https://sci-hub.se/https://pubs.acs.org/doi/10.1021/ja01043a...
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Interesting, so according to that paper it looks like the complex that forms with excess beta alanine present is
[Cu(β-Ala)3(H2O)2]-
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I want to make cobalt taurine salt. Will the metal bind to the amine group and water molecules? Because there are more oxygen on sulfur. Will it not
prevent?
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Sulfonic acids are terrible ligands: worse than carboxylic acids. If you reacted cobalt carbonate with taurine, yes, the sulfonic acid would be
deprotonated and you'd have the cobalt salt, but as far as how the ions would arrange themselves, well, amines are much more strongly coordinating
than sulfonic acids, so you'd likely have a structure with the amine nitrogens coordinated to the cobalt ions and the sulfonic acids sticking out
further away. Also worth noting that cobalt(II) amine complexes are quite easy to oxidize to their corresponding cobalt(III) complexes. You can do it
with moderately concentrated hydrogen peroxide. You should give it a try.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Quote: Originally posted by Texium (zts16)  | | Sulfonic acids are terrible ligands: worse than carboxylic acids. If you reacted cobalt carbonate with taurine, yes, the sulfonic acid would be
deprotonated and you'd have the cobalt salt, but as far as how the ions would arrange themselves, well, amines are much more strongly coordinating
than sulfonic acids, so you'd likely have a structure with the amine nitrogens coordinated to the cobalt ions and the sulfonic acids sticking out
further away. Also worth noting that cobalt(II) amine complexes are quite easy to oxidize to their corresponding cobalt(III) complexes. You can do it
with moderately concentrated hydrogen peroxide. You should give it a try. |
Thank you so much. Can you give me 2 and 3 complexes structures?
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
This is best what I found. It doesn't tell you anything about structures, but at least about basic formula and stability of these complexes:
https://core.ac.uk/download/pdf/207493218.pdf
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
thanks. I have already downloaded this file. From what I saw I downloaded everything but the structure was nowhere to be found. Neither sci-hub could
help me
|
|
|
Texium
Administrator
       
Posts: 4581
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Without a crystal structure, knowing the exact structure of the complex with certainty isn't really possible, but cobalt in either oxidation state
typically forms octahedral complexes with amines, so my hypothesis is that you would end up with something along these lines if you start from
cobalt(II) carbonate:
H4[Co(Tau)6] for cobalt(II)
and
H3[Co(Tau)6] for cobalt(III)
You could also try obtaining the alkali metal salt of the complex ion by partially neutralizing the taurine before reacting it with your cobalt
carbonate. Whatever the exact structure may be, with an excess of taurine, you will likely have six taurines coordinated to each cobalt ion through
the amine nitrogens. I doubt that the sulfonates would be good enough ligands to displace any amines from the first coordination sphere.
Keep in mind that I am not an expert in inorganic chemistry by any means, and these conjectures are based on a limited, mostly academic understanding
of how this stuff works.
|
|
|
vano
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Thanks. Today i bought 4% solution of taurine and i will try some complexes.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
I've been struggling to get a dry-ish chromium B-alanine salt. I tried some 4 or 5 different ways up to now; a violet colored product salt is the
common result. But it is very hygroscopic and worse it forms a sticky mess with water, methanol, and glacial acetic acid. Still ongoing and I will
eventually post all the details.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Hmmmm. This isn't entirely related, but somewhere I glimpsed a reference that inferred, complexing with copper may provide some "Protection" for
Amino-Acids.
Can these complexes, act as "protecting groups" in some circumstances? Sequestering Amino-groups, away from undesirable suitors?
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Chromium:
I spent hours trying to get a reasonably dry Chromium B-alanine complex. I wont detail all the attempts as it will be pages but some observations:
- The fact that something with a violet color forms is a common link between all the attempts.
- Cr(OH)3 boiled with b-alanine for some 12 hours seems to yield 2 products: a grey insoluble ppt and a violet very soluble product that seems to have
a low melting point, turns more white on the steam bath, but once cooled slowly becomes violet again. Photo further down. But there also always seems
to be unreacted Cr(OH)3 left over.
- B-alanine with Cr(NO3)3 boiled - no obvious reaction.
- B-alanine boiled in water with chromium acetate in (I am not sure of the formula; it can be various) yield vinegar smelling fumes and a very dark
(beautiful) purple syrup which is near impossible to get a dry salt from. This syrup boils at near 120C so my usual solution of boiling it out in
toluene don't work as toluene has a lower bp (I dont have xylene at the moment). This purple product is quite soluble in methanol. One attempt
involved boiling on the steam bath until the sticky syrup did not lose weight any more, then dissolving in methanol in batches and pouring the
methanol solution into boiling toluene. My hope being that the methanol would boil off leaving the product as a powder in the toluene. This worked to
an extent as I got some crystals; I left them to dry and then next morning they were a wet sticky mess - its also quite hygroscopic.
- Adding b-alanine to NaOH to produce the sodium complex and then adding a chromium iii chloride to this immediately produces a black ppt. And.....the
filtrate is violet!
- I also found very little info about chromium b-alanine complexes online which does not help with working out stoichiometry. So I ran some
experiments a few times with different ratios.
Chromium hydroxide and b-alanine at the start of the experiment.

Hydroxide and b-alanine after some 12 hours boiling and then left overnight to settle. Green unreacted hydroxide, very fine grey suspensded powder,
and violet supernatant solution.

The hydroxide reaction filtrate.

The salt extracted from the hydroxide filtrate.

The black salt that ppt out when b-alanine was first reacted with NaOH and then with CrCl3.

The dark purple thick liquid from reacting chromium acetate and b-alanine and many, many hours on the steam bath.

Trying to boil out the above in toluene - it forms a sticky plasticky mess! But some (violet) crystals can be seen in the bottom of the beaker.

|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Gmelin's

|
|
|
| Pages:
1
2 |