| Pages:
1
2 |
Peaches
Harmless

Posts: 2
Registered: 30-6-2019
Member Is Offline
|
|
Acetic Anhydride from Zinc Acetate?
TL;DR: I may have made acetic anhydride without chlorinating agents or phosphorous compounds using accessible chemicals.
I found a paper that details how to make basic zinc acetate. While basic zinc acetate isn't a very interesting chemical, the reaction the authors used
to make it was. In the reaction four molar equivalents of anhydrous zinc carboxylate are heated under vacuum to yield one molar equivalent of
carboxylic acid anhydride and one molar equivalent of basic zinc acetate. At first, this sounds horribly inefficient, and it is... But I speculate
that you can recycle the basic zinc acetate back into regular zinc acetate by acidifying it with acetic acid. You might be able to do several runs
before having throw out the zinc acetate.
1) The acetic acid is formed 4 Zn(CO2CH3) ---> Zn4(CO2CH3)6O +
(COCH3)2O
2) The zinc acetate is regenerated Zn4(CO2CH3)6O + 2 HCO2CH3 ---> 4
Zn(CO2CH3) + H2O
I decided to try this out for myself as synthesizing carboxylic acid anhydrides without chlorinating agents would be a very valuable reaction for
myself and the amateur chemist community; So I set to work and made some zinc acetate crystals by refluxing zinc oxide with 2 molar equivalents of
acetic acid. A little bit of water was also added to better solvate the reactants.
ZnO + 2 HCO2CH3 --->Zn(CO2CH3)2
After 3 hours of refluxing, the reactants were taken off heat and allowed to cool. The result can be seen in the first attached picture. The zinc
acetate crystals were vacuum filtered and placed in a beaker. The beaker was heated to 150oC for 5 hours with regular agitation to dry
them. The result was a fine, free-flowing, bone dry powder. For the next step, all involved equipment was rigorously dried before use. An Erlenmeyer
flask was charged with anhydrous zinc acetate. A vacuum distillation apparatus was assembled around the flask. Do note that using a water aspirator is
not advisable due to the moisture sensitive nature of this reaction. I used a shop vac and as you will soon read, this was a blunder too. In any case,
I pulled a vacuum and warmed up my hotplate to 250oC. The reaction sputtered real bad. Some of the zinc acetate found its way up to the
still head and some even into the receiving flask. The whole reaction was kinda sketch, and the sputtering kinda spooked me. The heat was held for 6
hours as was consistent with the paper, but no distillate came over. I was very upset at this point because I THOUGHT the reaction didn't work. I
unplugged my hotplate, let everything cool, and proceeded to disassemble the apparatus. But when I took the stopper off of the still head, I was
struck with the horrible stench of acetic acid. You know the smell. I concluded that I did make some acetic anhydride, but due to the high reaction
temperatures, the acetic anhydride vapor was sucked up the vacuum line before it had a chance to condense. The vacuum I was using was 70 mcmHg, which
I think was too much. Also the vacuum pump oil reeked of acetic acid; I really hope it didn't damage my pump.
So in conclusion I'm not sure if I made acetic anhydride or not... but based on the horrible pungent acetic acid like smell, I think I did. If someone
else wants to verify that would be awesome!
Here is a link to the paper.
Attachment: Freshly prepared zinc acetate.HEIC (1.4MB)
This file has been downloaded 480 times
Attachment: Zinc acetate sputtering.HEIC (1.6MB)
This file has been downloaded 431 times
Attachment: Zinc acetate dust in reciving flask.HEIC (1.7MB)
This file has been downloaded 404 times
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Peaches and welcome to the forum!
Interesting. I thought that such acetate salts (Ca2+, Sr2+, Ba2+, Zn2+, Mn2+) produce acetone and metal carbonate on heating. But you used vacuum and
temperature kept at 250 C on hotplate so maybe 200 C inside reaction flask to prevent that reaction.
What is 70 mcm Hg? Milli centimeter? I have 2-stage vacuum pump with granted vacuum 0,3 Pa and at full vacuum it usually decreases boiling point by
approx. 150 C (it depends on the compound distilled, condition of the pump, freshness / purity of oil and oil vendor, quality of ground glass joints
and quality of vacuum grease applied to the joints e.g. Korasilon is good etc).
For adjusting vacuum level you need a vacuum control valve, if the vacuum level is too high (low absolute pressure) you allow a little of air to be
sucked through the valve. For trapping low boiling liquids you need to connect a cold trap between the distillation apparatus and the pump. This is a
vessel surrounded by something very cold (dry ice + acetone, or for special purposes even liquid nitrogen) in which the vapor condenses and does not
enter you vacuum pump.
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
| Quote: |
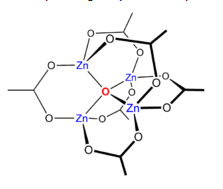
Zinc(II)acetate dihydrate loses its water of crystallization at 100degreesC to give Zn(CH3COO)2
Heating Zn(CH3CO2)2 in a vacuum results in a loss of acetic anhydride, leaving a residue of basic zinc acetate, with the formula Zn4O(CH3CO2)6. This
cluster compound has the tetrahedral structure shown below.
|
Koyama, H.; Saito, Y. (1954). "The Crystal Structure of Zinc Oxyacetate, Zn4O(CH3COO)6". Bull. Chem. Soc. Jpn. 27 (2): 112–114.
https://www.journal.csj.jp/doi/10.1246/bcsj.27.112
https://sci-hub.tw/10.1246/bcsj.27.112
/CJ
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
This is most interesting to me and deserves further effort.
Just found two half asssed attempts here, one from 2talltman and another by aga.... Waffles seemed to think he had success with silver acetate but I
guess it is expensive.
Link for anyone who's interested -
https://www.sciencemadness.org/whisper/viewthread.php?tid=9&...
/CJ
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
On the acetic anhydride thread this concept has been brought up a couple times and each time it gets shot down (justifiably). It's interesting to see
experimentation with this method. Good stuff 
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
Here’s some important information, it seems that it needs to be done at a max of 250c and without the presence of oxygen according to
https://opus.lib.uts.edu.au/bitstream/10453/20439/2/02Whole....
Basic zinc acetate was never the coproduct, the coproduct is zinc oxide, a side reaction which is where the failure comes from, you’re going after
the wrong reaction.
[Edited on 5-2-2021 by pip]
[Edited on 5-2-2021 by pip]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
If I cared enough to try I’d try 200c with an inert atmosphere and no vacuum.
But ketene seems easier, for now at least. It’s predictable.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Interesting work Peaches! Didn't you use a condenser/receiver beyond the stillhead? I am surprised that the acetic vapours were carried away if you
were. If acetic anhydride was formed but remained in the flask I wonder if you could extract it was a non-aqueous solvent such as pet. ether?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The reason that most of us don't believe the zinc acetate paper is because their finding of acetic anhydride was based on a spectroscopic analysis
(EDIT: it was byok3y critiquing the NMR) which some more knowledgable members pointed out was equally consistent with a mixture of acetic acid and
acetone:
http://www.sciencemadness.org/talk/viewthread.php?tid=9&...
Also, the requirement of extremely stringent oxygen and moisture-free conditions makes it more technically demanding than it initially sounds. The
similar method with silver acetate is known to work in up to 70% yields, but the reaction will not tolerate more than a few ppm of atmospheric
moisture or essentially all of the Ac2O autocatalytically decomposes to Me2CO + CO2. Replicating these conditions in an amateur lab isn't
that much easier than performing a safe chlorination, since the requirements for atmospheric isolation are similar. To date, no amateur has
successfully prepared Ac2O from silver acetate even though this is a known method from reliable papers that isolated the product. By contrast, dozens
of people have succeeded with S2Cl2.
IIRC some members experimented extensively with copper acetates to no success, even though these are significantly easier to dry than the zinc
compounds.
[Edited on 6-2-2021 by clearly_not_atara]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
The paper I posted basically confirms the need for an oxygen free environment however it also seems to prove that it’s not simply a mistake in
interpreting an acetic acid acetone mixture incorrectly. It also claims that auto decomposition is from too high a temperature.
[Edited on 6-2-2021 by pip]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Quote: Originally posted by pip  | The paper I posted basically confirms the need for an oxygen free environment however it also seems to prove that it’s not simply a mistake in
interpreting an acetic acid acetone mixture incorrectly. It also claims that auto decomposition is from too high a temperature.
|
If you're referring to [attached], you should notice that the signal for AcOH is 100x stronger than the signal for Ac2O. This is the
highest proportion of Ac2O obtained by a PhD student who dedicated several years of his life to the study of zinc salt decomposition. Even if
I accept it all on its face, it's not encouraging.
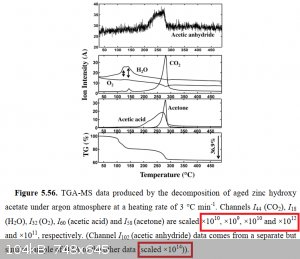
[Edited on 6-2-2021 by clearly_not_atara]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
I agree it’s not promising, my goal was pointing out that the reaction conditions being followed aren’t even the ideal ones. I don’t know if the
PHD student ever specifically went for acetic anhydride I didn’t care enough to read the paper past finding out what conditions are more optimal.
Personally I feel that the ketene method is so far superior that the time and effort spent researching this is better used to build an outdoor fume
hood with a vent stack that dumps well into the air above you. The fear of ketene is reasonable but that doesn’t mean we can’t plan around it.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You expect to get away with a 40' smokestack blowing ketene in your back-yard?
I don't get all of this obsession with "scale". You're not saving time by building a whole building devoted to ketene. Are you planning to
become some sort of heroin magnate?
There are plenty of methods that work: S2Cl2, NO2, BzH/TCCA, probably MeCN, and I'm still holding out hope for Cu(OAc)2+SO2.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Sometimes the scale of playing safe goes to extremes. Having a gas-tight apparatus with good scrubbing allows for quite ordinary fume hood practices
to keep everything safe.
Personally, I have access to a lathe, a mill and a tig welder. These can be used to churn out industrial standard stuff that can easily be proofed to
be absolutely gas tight, if it matters. In theory, all is needed is a distillation vessel, hot-torched tube for decomposition of acetone, collection
trap for unreacted acetone, and a bubbler with backflow valve to AcOH bath, and a further line to a scrub. Likely it could just be neutralized by
feeding the exhaust tube into an incinerator flame, just as oil refineries get rid of the remnants.
The real issue is as you said, building even a very small scale plant for ketene requires some investment for the subject, just to make some acetic
anhydride which you will need for...? It's not even so valuable in grey market that it would be worth the trouble to manufacture it for small scale
sales.
Usually, for the amateur, a need for small-ish amount arises, and for this metod it is apparently more convenient to do a single batch via chemical
synthesis. For some specific stuff, it can well warrant the trouble and the cost of reagents. If then so happens that more would be needed, conditions
could be reconsidered. Piloting a synthesis through the most available, not the most economical way is a general practice in many processes, and when
the concept is proven, the methods can be perfected by research and trial and error to find the sweet spot for every phase. I though am pretty sure
you ain't establishing a chem firm.
[Edited on 6-2-2021 by Fyndium]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
A bit dramatic huh? 40 foot smoke stack? A roof vent that’s ducted works equally well for a lot more uses than just ketene. If you don’t want a
vent in a garage that’s ok, but this method never has been viable, feel free to prove me wrong I hope you do.
The suggestion was simply for those scared of ketene. It is worse than cyanide and all. If you want to play around with milligram amounts zinc is for
you. If you think 250ml could only be used for heroin then I guess ok? I want it for chloroacetyl chloride. A nice bifunctional building block.
Spending 6 months to perfect a method that costs a lot of money in trial and error to get enough product to fill a dram container doesn’t sound
appealing. Dripping some acetone onto a hot wire and bubbling the gas into some acetic acid is just too easy and reliable. Spend a day doing it (yes,
scale) then not have to reproduce the experiment ever again or at least not for years. You can move on to the next project in a day.
I know it’s an amateur forum but I’m simply dumbfounded that scale is being criticized. No one is talking a liter of the stuff.
[Edited on 7-2-2021 by pip]
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I agree with pip. Some reagents, if needed, can be viable to make a larger batch and store it for years due to setup time.
Volatile non-persistent toxins are much easier to work with than heavy non-volatiles, organometallics etc which cannot be just vented out. First one
can disappear on its own in few hours to days at max or easily decontaminated with lye, bleach, ozone etc, latter can require tearing down a building.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Fyndium: sorry I don't own a lathe and usually think about stuff that works for people who don't have access to machinery.
There's nothing to
"perfect". S2Cl2 is well-known and you yourself are planning to work with chlorine so you know it's achievable.
Sure some other low-toxicity methods are more speculative cf. acetonitrile (although this one scales just fine), but we just talk about those because
there's more to talk about. 40 feet might be an exaggeration (although it's realistic if you count the horizontal pipe) but six months is a joke.
EDIT: also, you're talking about one hell of a complicated apparatus for a presumably gas-phase reaction between ketene and chlorine...
[Edited on 8-2-2021 by clearly_not_atara]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
What are you even going on about? Who’s talking about a lathe? The guy that casually mentioned that he’s lucky to have one? It’s not required to
build a vent hood.
Please spend all the time and money you want on this, I’ll await your groundbreaking advancements.
Cheer up and stop looking for fights to pick. No one suggests you need a lathe, no one wants gallons of the stuff, no one (hopefully) wants to make
heroin. And btw you could make meth or quaaludes just the same with AA along with who know what else it’s so versatile.
Let the OP experiment. If he wants to use the conditions in the paper I linked he could possibly get usable yeilds even if they’re not up to what I
personally feel would be a good use of time. If you want to work with other methods feel free, but you don’t need to come in and bring the mood
down.
We are hear to learn, experiment, and get advice from peers on better approaches to take. Not criticize, talk down, or just be grumpy.
[Edited on 8-2-2021 by pip]
[Edited on 8-2-2021 by pip]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Quote: Originally posted by Fyndium  |
Personally, I have access to a lathe, a mill and a tig welder. These can be used to churn out industrial standard stuff that can easily be proofed to
be absolutely gas tight, if it matters. In theory, all is needed is a distillation vessel, hot-torched tube for decomposition of acetone, collection
trap for unreacted acetone, and a bubbler with backflow valve to AcOH bath, and a further line to a scrub. Likely it could just be neutralized by
feeding the exhaust tube into an incinerator flame, just as oil refineries get rid of the remnants.
[Edited on 6-2-2021 by Fyndium] |
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
Yeah I edited to address that as you quoted.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Mostly, I'm trying to point out that you're giving dangerous advice that's also wrong. Ketene isn't the most practical way to make Ac2O, that's either
S2Cl2 or S+Br which A: have the most actual proven reports and B: don't require custom-built copper pipes. Before I called you out, you said in at
least two posts that the ketene method is a good way to make acetic anhydride, and... it isn't. Unless you have a personal machine shop, of course,
that's different.
Nobody should be encouraging anyone to make ketene because it's a bad idea and there are better ones. Seeing as you don't even seem to actually know
what the alternatives are, that goes especially for you. If you want to play with ketene, I can't stop you, obviously.
| Quote: | | If he wants to use the conditions in the paper I linked he could possibly get usable yeilds even if they’re not up to what I personally feel would
be a good use of time. |
No, there is absolutely no way that any amateur (and for that matter, probably not any professionals) is going to isolate any acetic anhydride by
heating zinc acetate, period. You should know that because we both went over the data in said paper.
| Quote: | | I’ll await your groundbreaking advancements. |
This is another example where you don't seem to get the point, I'm talking about methods that have already been proven to work,
there's nothing to advance, and furthermore I don't want Ac2O anyway.
| Quote: | | Not criticize, talk down, or just be grumpy. |
Discussion without criticism isn't appropriate for dangerous activities. If you want to discuss without being criticized, you can go post in a forum
about anime or something.
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
Waste of time, waste of chemicals, I’ll stick with ketene. But that’s opinion. Acetone is cheap, acetic acid (or just water) is cheap. There’s
no multi step process unless you count distilling acetic acid from water or distilling the anhydride.
I already said the zinc method was also a waste of time, but lots of wastes of time turn out to be useful by someone going through trial and error. I
won’t discourage him from trying, if I did I do apologize, I will say the same thing youre saying as far as the chances of success are very low.
It’s his time to waste and he’d be a little bit of a hero if he can figure it out. I’m not aware of the author of the paper I posted
specifically going for AA as his product, it’s possible with optimization he can get a couple percent who knows maybe even 10% or more. Likely,
probably not, possible sure.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Here's another opinion: it's better to waste a million dollars worth of chemicals than a single human life. A well-built ketene setup will
produce a decent amount of AA. A badly built ketene setup will produce a dead body and a story on the six-o-clock news about the dangers of amateur
chemistry and (hopefully not) the "Sciencemadness website" that encouraged the decedent to build it.
I still doubt you could build a ketene setup faster than gassing some sulfur with chlorine and react the product with acetic acid. I believe Fyndium
could build a ketene setup because he demonstrated in the first sentence he understood what it requires.
Look, ketene is achievable, but it absolutely should not be your first high-temperature gas-phase reaction. Likewise, you should not
attempt to pilot the first boat you build across the Atlantic Ocean. If your plan is to go from "never performed a gas-flow reaction above 100 C" to
"successfully made ketene" and you think you're going to save time and money relative to chlorinating sulfur, you're out of your mind.
https://www.ncbi.nlm.nih.gov/books/NBK224928/
"Like phosgene, the pulmonary effects of inhalation exposure to ketene may be manifested in the absence of direct irritation by
ketene or its breakdown product, acetic acid."
[Edited on 9-2-2021 by clearly_not_atara]
|
|
|
pip
Hazard to Others
  
Posts: 109
Registered: 19-9-2008
Member Is Offline
Mood: No Mood
|
|
There must be something wrong with you. You just look for arguments, get help man.
Bubbling ketene twice and venting the tiny amount that makes it past both containers with a high powered fan will be more than adequate.
I have a feeling you’ve never actually done it.
[Edited on 9-2-2021 by pip]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
It would have cost you nothing to not say that.
|
|
|
| Pages:
1
2 |