Steve s
Harmless

Posts: 46
Registered: 20-2-2019
Member Is Offline
|
|
Containing iodine as liquid
As per subject.
I know this is not possible under normal pressure and temperature but i'm thinking it might be if a low enough pressure can be achieved.
I'm thinking thick walled quartz tube containing a sufficient quantity of elemental iodide ''sublimated'' under vacuum and heat before quickly sealing
the tube.
Hoping as it cools some might stay as liquid right down to room temp or better still when warmed to just above.
Can't find any info on this so i'm guessing that low of a pressure is not obtainable, don't want to waste my time and iodine on this if it is
'impossible' any thoughts ??
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Nile Red did a good demo on iodine, busting the myth that it doesn't melt at standard pressure.
https://www.youtube.com/watch?v=dPIaEWd8zf4
Looking at the phase diagram though, I don't see that it is possible to achieve what you are after.
|
|
|
Steve s
Harmless

Posts: 46
Registered: 20-2-2019
Member Is Offline
|
|
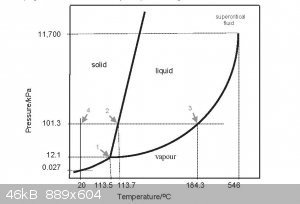
It was actually that very video that got me thinking about this, yes unless i'm reading it wrong 0.027Kpa at 20c it would be sublimating not melting,
then the pressure will rise the more it sublimates.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
No mater the pressure it can't be a liquid at less than 113.5 C.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4364
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
You want a high pressure if you want it to melt rather than sublime.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 564
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Oh, it sure can. Liquids commonly undercool in absence of good crystallization nuclei.
But you cannot melt a solid into an undercooled state.
How do you suppress crystallization nuclei in molten iodine to prompt iodine to undercool?
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
If you ampule iodine under vacuum you can liquify it by heating. Depending on the vacuum it will liquify somewhere above 113 degrees.
|
|
|
unionised
International Hazard
    
Posts: 5129
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Steve s  | | It was actually that very video that got me thinking about this, yes unless i'm reading it wrong 0.027Kpa at 20c it would be sublimating not melting,
then the pressure will rise the more it sublimates. |
Water at -35C (or thereabouts) has a vapour pressure of 0.027 KPa
Do you think that somehow stops it ever being a liquid?
|
|
|
Steve s
Harmless

Posts: 46
Registered: 20-2-2019
Member Is Offline
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by Steve s  | | It was actually that very video that got me thinking about this, yes unless i'm reading it wrong 0.027Kpa at 20c it would be sublimating not melting,
then the pressure will rise the more it sublimates. |
Water at -35C (or thereabouts) has a vapour pressure of 0.027 KPa
Do you think that somehow stops it ever being a liquid? |
It would appear that in these conditions it will sublimate before it melts (liquefies) which is still pretty cool but not as cool as being able to
warm it enough to melt it with just the heat from ya hand.
|
|
|
Steve s
Harmless

Posts: 46
Registered: 20-2-2019
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | If you ampule iodine under vacuum you can liquify it by heating. Depending on the vacuum it will liquify somewhere above 113 degrees.
|
Again still pretty cool but important to make sure you've got a decent vacuum in the ampoule before heating it once sealed
|
|
|
Yttrium2
Perpetual Question Machine
    
Posts: 1104
Registered: 7-2-2015
Member Is Offline
|
|
how is it liquid it iodine tincture? alcohol?????????
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Think of the different physical states.
The question is, how to contain iodine in its molten state, which is obviously a problem.
But that should be apparent when reading the thread.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 564
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Yes.
|
|
|
unionised
International Hazard
    
Posts: 5129
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Steve s  |
It would appear that in these conditions it will sublimate before it melts (liquefies) which is still pretty cool but not as cool as being able to
warm it enough to melt it with just the heat from ya hand. |
As has been pointed out, you can melt it in a test tube; nothing complicated is needed.
But it simply won't melt from the heat of your hand any more than steel would, and for the same reason.
The lowest you can get (pure) iodine to melt is the triple point at 113.5 °C at 12.1 kPa
[Edited on 5-7-20 by unionised]
|
|
|