AlbinoMoose308
Harmless

Posts: 4
Registered: 27-1-2015
Location: TN, USA
Member Is Offline
Mood: No Mood
|
|
barrels of solvent, not many uses... any ideas?
So I happen to have access to +40 gallons of 2 different organic solvents and was wondering if there's anything interesting/useful I can do with them.
The solvents are 1-Butoxy-2-Propanol and 2-Butoxyethanol. They work wonders for degreasing but surely those butyl groups are just begging to do
something fun! 
Acquiring a lab is difficult when you're dead-set on not spending a penny
Chlorine trifluoride should be officially designated "liquid fire"
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
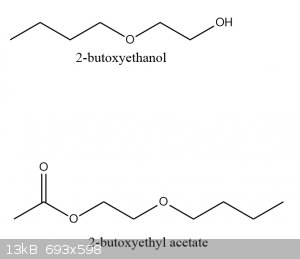
Make acetate esters by fischer esterification. see if they have an interesting smell.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Can they be hydrolyzed like esters into there respective alcohols?
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Lucky! like drac suggested you could hydrolyze the ether to ethylene glycol and butanol (both useful in large quantities), and the ester to the acid
and ether. I think you can selectively hydrolyze the ester with a strong base and then the ether with an acid after workup.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I'd consider selling them in 1 litre bottles via eBay then use the cash to buy more interesting stuff 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
I'd agree selling some or trading some is definitely a good idea. Also hydrolyzing some into other products to play with opens up possibilities as
well.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I seem to recall that hydrolysis of an ether is pretty difficult. You may have better luck cleaving them into alkyl halides.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Ethers can be hydrolyzed by HBr into alkyl halides. the products will be 1-bromobutane and 1,2-dibromoethane
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Am I right in guessing that hydrochloric acid might be too weak to make the chlorides
Anyway the alkyl halides sound more interesting and they further open up possibilities.
I think straight butanol or secbutanol can be used as a petrol substitute without modification of the car.
[Edited on 4-6-2020 by draculic acid69]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Making the chlorides will just be very slow.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Could it be helped along with zncl2 or something?
|
|
|