Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Was shipped wrong item, got a bottle of glucose instead of choline chloride. Anything cool I can do with glucose?
It was only $7 and dont wanna go thru the hassle of amazon returns.
Im currently in biochemistry I. What kind of organic reactions can I do with glucose? Cleavage of the cyclic hemiacetal bond to form linear glucose?
It has a ton of alcohol groups. If I oxidized with dichromate, would I get some weird compound like a carbon chain with a ketone group at every carbon
of the chain? Could I do a fisher esterification with acetic acid to acetylate every hydroxyl group?
[Edited on 18-4-2020 by Cou]
[Edited on 18-4-2020 by Cou]
|
|
|
dawt
Hazard to Self
 
Posts: 74
Registered: 9-5-2016
Location: EU
Member Is Offline
Mood: fluorescent
|
|
Anything drastic (i. e. Fischer esterification etc) is going to yield you tar and a whole bunch of dehydrated products. Sugar chemistry tends to be
really messy even under the best circumstances. If you want to isolate any product I'd look into enzymatic reactions instead! Depending on
which enzymes you can get your hands on you can make all sorts of useful stuff: Check out glycolysis amd the Krebs cycle.
Otherwise I'd get a couple of amino acids and do some Maillard reactions in test tubes. Vary the reaction conditions, e. g. temperature, pH, solvent
(or no solvent at all) and note the differences in smell, reaction rate etc.
Edit: Since glucose is a reducing sugar you might also want to test it on some inorganics and see which ions it can mess with. This could yield you
anything from gluconic acid to carbon dioxide, depending on the oxidising power of your ion under the selected conditions.
[Edited on 2020-4-18 by dawt]
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
If you have never done the blue bottle reaction, you should. And there are some variants.
The manganese chemical chameleon works differently with glucose instead of sucrose. That might be a worthy investigation.
You might try polymerisation. You aren't going to get anything that you can analyse but I bet you could find something interesting and quantifiable.
Then there is tons you could do biologically.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2825
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Reduction of glucose gives sorbitol.
Sorbitol can then be converted to sorbitan or isosorbide by acid-catalyzed dehydration. These are much more "well-behaved" chemicals that can become a
number of things.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
actually the bottle was sucrose, not glucose. I was a little frustrated at wrong item and set the bottle at front door, didn't check name thoroughly.
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Fermentation and distillation?
|
|
|
brubei
Hazard to Others
  
Posts: 188
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
acidic hydrolysis of glucose yield to Hydroxymethylfurfural, a flavouring agent (but do not eat it !)
https://en.wikipedia.org/wiki/Hydroxymethylfurfural
I'm French so excuse my language
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Wow, sucrose, that's about as disappointing as it gets from a chemistry standpoint. I think unionised's suggestion is the most practical. That, or
combine it with butter, eggs, and flour with a catalytic amount of sodium bicarbonate and synthesize some cookies!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2825
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
You can make levulinic acid from sucrose!
But you should complain. You bought a high value product and they sent you something cheap. Glucose is at least kind of interesting.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Name of the company is HiMedia. they are an amazon seller that i have bought propionic acid and benzophenone from, prime delivery, but this time they
messed up and they have bad customer service.
https://www.amazon.com/gp/product/B00DYO6706/ref=ppx_yo_dt_b...
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Interestingly when you look at the reviews, they apparently had people experiencing almost the exact opposite problem!
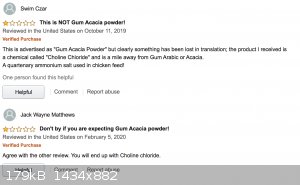
Edit: those are the only two reviews. Presumably, the item listing used to say gum acacia powder, and it was changed to choline chloride later.
[Edited on 4-18-2020 by Texium (zts16)]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
What did you want to make from choline chloride, was it more available form of choline such as alpha gpc or cdp-choline?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Quote: Originally posted by mackolol  | | What did you want to make from choline chloride, was it more available form of choline such as alpha gpc or cdp-choline? |
A mixture of choline chloride and glycerin can be used to extract alcohol from impure ester, e.g. the mixture of 1-nonanol and nonyl acetate after
working up a fischer esterification. Just mix with the deep-eutectic solvent, the pure ester separates from the DES/alcohol layer. https://www.sciencedirect.com/science/article/pii/S004040391...
in a thread in the organic chemistry forum i brought up a problem with synthesis of esters from acetic acid and long chain alcohols by fischer
esterification: even with a large excess of acetic acid, the reaction doesn't use up all the alcohol. long alcohols like 1-nonanol can't be extracted
with water, can't be separated by distillation, and they contaminate the smell of the ester.
Using acetic anhydride or acetyl chloride is one way to use all the alcohol, but they are both hard to get, expensive, and doesn't solve the problem
for formate esters since formic anhydride and formyl halides are both unstable.
the steglich esterification goes to completion, but choline chloride and glycerin are more readily available and cheaper than the catalysts used in
steglich esterification. Industry prefers fischer esterification because the lower yield outweights the cost of reagents that can esterify to
completion.
The dean-stark method doesn't work for making acetate esters, since acetic acid and toluene form an azeotrope below the boiling point of toluene.
I think the best way to do this is to do a fischer esterification with large excess of acetic acid (a very cheap chemical) and purify the crude ester
with the deep eutectic solvent.
[Edited on 18-4-2020 by Cou]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Very interesting and very useful Cou, thanks for sharing. I didn't know that.
|
|
|
Metacelsus
International Hazard
    
Posts: 2543
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by Cou  | Name of the company is HiMedia. they are an amazon seller that i have bought propionic acid and benzophenone from, prime delivery, but this time they
messed up and they have bad customer service.
|
I've bought stuff from them before (crystal violet and EDTA). Too bad they messed up your order.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2816
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
I would use it in coffee... Just sedn them a note and ask them to fix it. Any reasonable company will fix their mistake, if not, file a complaint
or leave more bad feedback. That will keep them from messing up others.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Just received choline chloride. it has a weak fishy amine odor. $30 for this bottle of 500 g, but it's still a lot cheaper than the chemicals used in
esterification reactions that go to completion, such as steglich esterification and using acid chlorides, anhydrides.

[Edited on 19-4-2020 by Cou]
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Texium (zts16)  | | Wow, sucrose, that's about as disappointing as it gets from a chemistry standpoint. I think unionised's suggestion is the most practical. That, or
combine it with butter, eggs, and flour with a catalytic amount of sodium bicarbonate and synthesize some cookies! |
I can go with the cookies, or a cake. Or just use it to sweeten your tea or coffee
|
|
|