| Pages:
1
2 |
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Attempts to make calcium iodide
1st attempt: 2.5g calcium granules in 150ml DCM, stir. Decided to add iodine in small amounts in case of an exothermic reaction. Add 1g I2, solution
colour dark. Bring to boil. After one hour 30 minutes no sign of iodine consumption, no colour change, stop.
2nd attempt: Decided, against my gut feeling, to try in acetone because I read that CaI2 is soluble in acetone. Started with 1g Ca and 2g I2 in 150ml
acetone, heat and stir. After 30 minutes the solution seemed to clear up a bit. Add 2g I2. After another hour there was no colour change, stopped and
filtered hot to see what I had. The solution just appeared to be iodine in acetone. On the filter paper was a lot of white powder and some unreacted
calcium. The white powder did not dissolve in water and shaking it with a grain of lead nitrate gave no ppt of lead iodide, I suspect this powder was
calcium oxide.
While cleaning the glassware something started to sting my eyes! No unusual small, but definitely something other than acetone. Went and washed my
face and tried again – same issue. Quickly place everything in a bucket of water. What could have formed?
3rd attempt: decided to try in ethanol as CaI2 is soluble in alcohols. The ethanol I had was clear ‘methylated spirit’ which was supposed to be
95% ethanol and 5% water.
0:00 1g Ca and 2g I2 in 150ml ethanol, stir only. Dark solution.
1hr15min - no change in colour, switch on heat and bring to gentle boil.
2hr - colour change to light brown. Add 2.5g I2, dark solution.
While waiting for a colour change, I realised that some of the calcium could have reacted with the 5% water. I also looked at my calcium again and saw
that it was heavily oxidised, could be as much as 50%. Thus decided to add more calcium.
2hr40min: Add 2g calcium, and increase ethanol to 180ml in case the solubility of CaI2 in ethanol was very low (I could not find figures).
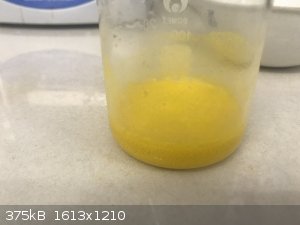
3hr30min: solution colour changed to yellow! Continued another 15 minutes but no further change. See photo.
- Filter hot. Again a lot of white powder on the filter paper that is not soluble in water, also some unreacted Ca.
- Boiled down to some 10ml, colour became darker yellow towards brown. See photo.
- Transfer to a crucible and placed in desiccator on NaOH……and found my $15 vacuum pump was destroyed by the last use with DCM vapours. The
crucible stayed put in the desiccator for 24 hours while I looked for another pump. I saw it was gradually becoming darker, not sure if light was
causing it to break up and freeing iodine.
- Got a professional air-conditioning service vacuum pump. Pulls close to a perfect vacuum, see photo. Sucked a vacuum on the desiccator and re-vacuum
every few hours during the days. Desiccator sealing well, still 500mb vacuum left after 12 hours overnight.
- The volume decreased, and it became a thick syrup but then after another 48 hours it was still very wet.
- Saw I had a big stopper that fitted my 100ml beaker. Did a test to see if the beaker could withstand a vacuum, by pulling a vacuum on the beaker for
10 minutes while it was placed inside another beaker with boiling water. Seemed ok.
- Transferred the syrup to the small beaker, pull vacuum with the beaker in boiling water. See photo. Some more ethanol boiled off but after an hour
this slowed down and it was still not dry.
- Placed the small beaker directly on a hot place while pulling vacuum. See photo. This got it dry, and the colour lightened as some iodine sublimed
and condensed in the hose.
- Let the beaker cool, removed the vacuum, and removed the cork (with difficulty). Left in the beaker was a white-yellow-brown mix. See photo.
Scratched out as much as I can and placed in a vial, 3 gram. See photo.
- There was still a very hard layer in the bottom of the beaker and I was this was hygroscopic and getting wet – I added a small amount of water
that dissolved the layer in seconds, forming a yellowish solution, see photo. I then added lead nitrate solution to this and got lead iodide ppt. See
photo.
Seems I got some calcium iodide albeit with too much free iodine to my liking. Not sure if it is a more complex salt, but it does give the expected
ppt wit lead nitrate.
I will do a second run when I have time free and change a few things to see if I get a purer product. Will file an update in due course.
|
|
|
Tsjerk
International Hazard
    
Posts: 3037
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You probably made iodo-acetone, which is a strong lacrimator
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Based on this source http://calcium.atomistry.com/calcium_iodide.html (extracted from old chemistry journals) to quote commentary on CaI2:
"The salt may be prepared by the action of hydriodic acid on lime or pure marble, or, instead of the acid, iodine and a reducing agent may be used -
for example, iron filings or phosphorus. "
-------------------------------
I would like to comment on the possible mechanics of iron acting on aqueous iodine as a path to HI:
I2 + H2O = H+ + I- + HOI
Now, with a transition metal acting on HOl, I would expect a complex reaction system starting with a fenton-type reaction as occurs with HOCl (see,
for example, https://www.researchgate.net/publication/275818872_Influence... and, I suspect, also with HOBr and likely HOI):
Fe + 2 HOI --> Fe(ll) + 2 •IOH-
Fe(ll) + 2 HOI --> Fe(lll) + 2 •IOH-
Which consumes HOI, moving the iodine water equilibrium above to the right and introduces a radical anion. Further, assuming a parallel pH-sensitive
breakdown to the •ClOH- radical anion:
•IOH- -> •I + OH- (pH likely below 5)
•I + •I -> I2
which recycles elemental iodine.
Also possible, in the presence of a high concentration of iodide, the formation of a problematic stable radical anion:
•I + I- -> •I2-
At higher pH, I would expect the formation of the hydroxyl radical:
•IOH- -> I- + •OH (pH likely above 5)
and at elevated pH, an acceleration of the disproportionation reaction of HOI to iodate (see, for example, https://www.osti.gov/servlets/purl/5334892-Pulvr4/ ) with iodates also generally displaying reduced solubility:
3 HOI = 2 HI + HIO3
And the cited equilibrium reaction:
5 I- + IO3- + 6 H+ <--> 3 I2 + 3 H2O (see, for example, https://www.sciencedirect.com/topics/chemistry/iodate )
More radical reactions including the reverse radical reaction:
I- + •OH -> •I + OH-
Note, the atomic iodine radical could also be removed by interacting with •IOH- as follows:
•I + •IOH- -> I2 + OH-
which would be consistent, as I have previously noted on SM, with the •HO2 radical assisting in the liberation of elemental bromine (from, for
example, the stable radical anion, •Br2-).
See this related work with free bromine formation (and not complexed), 'Hydroperoxyl radical (•HO2) oxidizes dibromide radical anion (•Br2−) to
bromine (Br2) in aqueous solution: Implications for the formation of Br2 in the marine boundary layer', a work by Brendan M. Matthew, et al, fully
available (for free) at: https://agupubs.onlinelibrary.wiley.com/doi/full/10.1029/200... .
The above reaction system implies a possible formation of ferric iodide, but the latter does not exist (see explanation provided at https://chemistry.stackexchange.com/questions/51610/why-does...), just FeI2 which continues to feed a radical creation reaction, implying only a
limited amount of Fe presence may be required.
--------------------------------------------------
Note: pass any formed HI (g) into water and avoid prolonged contact with oxygen as hydrogen iodide is apparently relatively unstable:
HI (g) = •H + •I (see https://www.sciencedirect.com/topics/chemistry/hydrogen-iodi... )
The ability of HI to yield •H is the reason it is widely used in organic synthesis (same source).
•H + O2 --> •HO2
•I + •I -> I2
•HO2 + I2 --> H+ + O2 + •I2- (See Eq (1) at https://pubs.acs.org/doi/10.1021/j100398a045#)
[Edited on 11-1-2020 by AJKOER]
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi AJKOER thanks for that, I will have to do a lot of reading to even begin to understand these concepts...
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AJKOER  |
Now, with a transition metal acting on HOl, I would expect a complex reaction system starting with a fenton-type reaction as occurs with HOCl
[Edited on 11-1-2020 by AJKOER] |
You can't write a shopping list without including a fenton reaction.
There's no reason to assume anything more complicated than
Fe + I2 --> FeI2
Followed by FeI2 + CaCO3 --> FeCO3 + CaI2
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Incidentally, beware of using CH2CL2 as a solvent for reactive metal reactions.
|
|
|
Tsjerk
International Hazard
    
Posts: 3037
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Lion850  | | Hi AJKOER thanks for that, I will have to do a lot of reading to even begin to understand these concepts... |
No you don't, you can safely disregard these, and mostly any of AJKOER's comments as those of an obsessed old man.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
First, I cited an old prep for the salt in question, namely CaI2, not FeI2!
Next, I outlined the chemistry of how, I suspect, Fe is likely assisting in that prep in a possible aqueous setting (to be honest, I am not clear if
the wording implies a completely aqueous process). But, assuming it may, I have since added more source references for those searching for advanced
background material (and not the usual unsourced commentary) on such an experiment.
------------------------------------------------------
Unionised:
On your suggestion, which is one of the classic cited paths:
Fe + I2 --> FeI2
FeI2 + CaCO3 --> FeCO3 + CaI2
It is important to note (as confirmed in this reference https://books.google.com/books?id=Dv_F03cdKPUC&pg=PA472&... ) that FeI2 is prepared from warming dry solid iodine! Also, another source (http://www.docbrown.info/page07/transition06Fe.htm) comments "When iron wool is heated with iodine there is little reaction, a small amount of
iron(II) iodide is formed."
Then, the addition of water to FeI2 releases H+ (DocBrown again), which then could react with CaCO3, Ca(OH)2,...for an aqueous product.
Sorry, but I do not have any solid I2 around, but I do have a CVS Tincture of Iodine which is 2% I2, 2.4% NaI, 47% alcohol and the rest is 'purified
water'. So, any guidance on possible reactions from, say, adding Fe filings to this mix?
Let me guess, nothing that has a source reference.
There is one reference, however, citing that Iodophor is corrosive to iron and stainless steel (see https://www.sciencedirect.com/topics/biochemistry-genetics-a...).
I also did find further sources claiming "ferric and cupric salts are reduced in acidic solution by the iodide ion, to form free iodine" per https://patents.google.com/patent/EP0172984A1 and also DocBrown. This supports my contention of a possible cyclic reaction system creating
radicals as long as there is a presence of HOI or aqueous I2 (and I-). Such a system could result in an increased presence of monoatomic iodine. The
latter has been referred to also as nascent iodine, and has associated benefits per, for example, this world patent https://patents.google.com/patent/WO2016175727A1/en .
Given that Iodophors can display life-saving disinfecting properties, any enhancement with say iron (or even copper) ions may have significant
consequences. Perhaps one should think twice as to whether "you can safely disregard" my comments.
[Edited on 12-1-2020 by AJKOER]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
NaI reacts with acetone for sure, I wonder if other iodides do. Acetone does react with calcium and presumably calcium hydroxide, and iodine/iodide +
iodate.
What is methylated about it then? You don't think that calcium would react with alcohols and water?
Is there a smell of iodoform?
My prediction in this thread is: things are not looking good for the new pump.
Whatever this is, it's not what the world needs.
[Edited on 11-1-2020 by S.C. Wack]
|
|
|
mhz4.77
Harmless

Posts: 4
Registered: 12-11-2019
Member Is Offline
|
|
Vis-a-vis Iodophor corroding iron and Stainless Steels.
For quite a few years we used Iodophor solutions for sanitation rinses and soaks. I can confirm it will corrode unprotected iron, but its action on
stainless steel seemed quite unpredictable. Some stainless steels would pit unpleasantly, others would show no signs of damage at all. I don't know
what the actual grades were, but they were mostly cutlery stainless steels.
6==NaN
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Hey lion850. Firstly iodine bromine and chlorine are completely incompatible with acetone as haloacetones are formed.they are teargas agents and
aren't friendly chemicals unless you're in a full spaceman suit with respirator.secondly i think DCM and alkaline metals are incompatible and by
calcium granules do you mean calcium metal? If u make the iodine into hydroiodic acid then mix it in water with the calcium metal you'll end up with
calcium iodide.maybe they can be mixed dry in a test tube and heated you'll get calcium iodide.
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by AJKOER  | First, I cited an old prep for the salt in question, namely CaI2, not FeI2!
Next, I outlined the chemistry of how, I suspect, Fe is likely assisting in that prep in a possible aqueous setting (to be honest, I am not clear if
the wording implies a completely aqueous process). But, assuming it may, I have since added more source references for those searching for advanced
background material (and not the usual unsourced commentary) on such an experiment.
------------------------------------------------------
Unionised:
On your suggestion, which is one of the classic cited paths:
Fe + I2 --> FeI2
FeI2 + CaCO3 --> FeCO3 + CaI2
It is important to note (as confirmed in this reference https://books.google.com/books?id=Dv_F03cdKPUC&pg=PA472&... ) that FeI2 is prepared from warming dry solid iodine! Also, another source (http://www.docbrown.info/page07/transition06Fe.htm) comments "When iron wool is heated with iodine there is little reaction, a small amount of
iron(II) iodide is formed."
Then, the addition of water to FeI2 releases H+ (DocBrown again), which then could react with CaCO3, Ca(OH)2,...for an aqueous product.
Sorry, but I do not have any solid I2 around, but I do have a CVS Tincture of Iodine which is 2% I2, 2.4% NaI, 47% alcohol and the rest is 'purified
water'. So, any guidance on possible reactions from, say, adding Fe filings to this mix?
Let me guess, nothing that has a source reference.
There is one reference, however, citing that Iodophor is corrosive to iron and stainless steel (see https://www.sciencedirect.com/topics/biochemistry-genetics-a...).
I also did find further sources claiming "ferric and cupric salts are reduced in acidic solution by the iodide ion, to form free iodine" per https://patents.google.com/patent/EP0172984A1 and also DocBrown. This supports my contention of a possible cyclic reaction system creating
radicals as long as there is a presence of HOI or aqueous I2. Such a system could result in an increased presence of monoatomic iodine. The latter
has been referred to also as nascent iodine, and has associated benefits per, for example, this world patent https://patents.google.com/patent/WO2016175727A1/en .
Given that Iodophors can display life-saving disinfecting properties, any enhancement with say iron (or even copper) ions may have significant
consequences. Perhaps one should think twice as to whether "you can safely disregard" my comments.
[Edited on 12-1-2020 by AJKOER] |
"First, I cited an old prep for the salt in question, namely CaI2, not FeI2!"
Yes, nobody said you didn't. And, if you had left it at that, your post would have been more helpful.
"Next, I outlined the chemistry of how, I suspect, Fe is likely assisting in that prep in a possible aqueous setting"
Yes, but you r "suspicion" seems to be based on your obsession with fenton reactions, rather than any actual evidence.
You cited a couple of references that confirm my assertion that iodine reacts with iron (no shock to anyone).
And you confirm the well known observation that halide ions increase the rate of corrosion of stainless steel.
And you repeated the well known observation that some transition metals ions (Cu, Fe) can oxidise iodide.
Well, one of those is a high school experiment- titration of copper sulphate by addition of excess iodine and reaction with thiosulfate.
Not relevant (because it';s not credible that significant Cu++ is present- if it was, it would immediately be stripped out of solution by reaction
with metallic iron) and Fe(III) will be absent for the same reason) and nothing new.
As for " Perhaps one should think twice as to whether "you can safely disregard" my comments."
Perhaps you should think twice before assuming the involvement of species like Cu++ and Fe(III) which can't be present while there is metallic iron
left.
Then you wouldn't make comments which should be disregarded on the basis of impossibility.
|
|
|
Tsjerk
International Hazard
    
Posts: 3037
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Thank you unionised for quoting AJKOER's "you can safely disregard".
I would never have seen his reaction as I stopped reading his shit a long time ago.
[Edited on 12-1-2020 by Tsjerk]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Thanks Unionised for your reveiw.
My further analysis, which is the heart of my atomic iodine presence, relies on the actual existent/stability of HOI (per conventional wisdom) to
engage in a fenton-type reaction in a timely manner. I am referring to my claimed reaction, which is appropriate for chlorine, bromine,..., namely:
I2 + H2O = H+ + I- + HOI
Note, the world patent I referenced cites only the key reaction equilibrium:
5 I- + IO3- + 6 H+ <--> 3 I2 + 3 H2O
------------------------------------------------------
Now, there is apparently already a lot of hype on the web relating to 'nascent iodine', so lets not further contribute.
[Edited on 12-1-2020 by AJKOER]
|
|
|
Tsjerk
International Hazard
    
Posts: 3037
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by AJKOER  |
Now, there is apparently already a lot of hype on the web relating to 'nascent iodine', so lets not further contribute.
[Edited on 12-1-2020 by AJKOER] |
LMAO
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Still waiting for anything to rule out the idea of I2(aq) or I3- being the initial oxidant.
[Edited on 12-1-20 by unionised]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | Still waiting for anything to rule out the idea of I2(aq) or I3- being the initial oxidant.
[Edited on 12-1-20 by unionised] |
I am impressed!
See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5529521/ .
I2 and I3- along with air and a metal are cited in this new generation of battery cells based on iodine REDOX chemistry.
|
|
|
woelen
Super Administrator
        
Posts: 8084
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Again, AJKOER makes things much more complicated than what is relevant for Lion850. If any of these reactions occur, then it will be at most in trace
amounts, possibly only transiently for a few nanoseconds  , but certainly not
long enough to be observed in any practical setup , but certainly not
long enough to be observed in any practical setup  . So, these effects can be
safely ignored for any practical means. . So, these effects can be
safely ignored for any practical means.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by S.C. Wack  | NaI reacts with acetone for sure, I wonder if other iodides do. Acetone does react with calcium and presumably calcium hydroxide, and iodine/iodide +
iodate.
Sodium iodide dissolves in acetone but doesn't react.acetone is a common solvent for NaI in rxns.
[Edited on 13-1-2020 by draculic acid69] |
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Per below: a recipe for FeI2, which is the cited starting compound to make iodides, per prior discussion above.
Just stir up a mix of Fe filings (in excess), water, Iodine, some alcohol (CH3OH) and a touch of NaI (optional to speed up the reaction), in a dish
open to air and sunlight. Or, Fe filings and a Tincture of Iodine (add beneficial CH3OH) in sunlight.
Logic: The electrochemistry makes rust which combines with I2 to form FeI3 (or I2(aq)/I3- attacks Fe per metal-iodine-air battery cell referenced
above). The sunlight converts this to FeI2 and I2. The released iodine further reacts with the iron. More details on the possible chemistry below
including iodinated organics.
-------------------------------------------------------------
Per this source https://www.ncbi.nlm.nih.gov/pubmed/31553594 to quote from the abstract:
"This study first reports that ferric chloride (FeCl3) can lead to the formation of iodinated coagulation byproducts (I-CBPs) from iodide-containing
resorcinol solution or natural waters. The unwanted I-CBP formation involved the oxidation of iodide by ferric ions to generate various reactive
iodine species, which further oxidize organic compounds. Although the oxidation rate of iodide by FeCl3 was several orders of magnitude slower than
that by chlorine or chloramine, most of the converted iodide under the ferric/iodide system was transformed into iodine and iodinated organic
compounds rather than iodate. Formation of four aliphatic I-CBPs was observed, and four aromatic I-CBPs were identified by gas chromatography
mass-spectrometry and theoretical calculation. Coagulation of iodide-containing waters with FeCl3 also produced I-CBPs ranging from 12.5 ± 0.8 to
32.5 ± 0.2 μg/L as I. These findings call for careful consideration of the formation of I-CBPs from coagulation of iodide-containing waters with
ferric salts."
The above implies to me especially in light conditions:
I- --> •I + e-
Fe(lll) + e- = Fe(ll)
Support: FeI3 is intensely photosensitive (see https://books.google.com/books?id=hpWzxTnQH14C&pg=PA265&... )
And, in the presence of organics (RH):
RH + •I = •R + HI
•R + •I = RI
I- + •I = •I2-
.....
And, to a reduced extent:
•I + •I = I2
with the formation of HI, there is a drop in pH, which reduces the pH sensitive formation of iodate at higher pH.
Support for the creation of transient iodine radical, likely in light conditions, is due to the reported creation of "iodinated organic compounds
rather than iodate" and the initial formation of "various reactive iodine species".
Note: Both FeI3 (and CuI2) exist but are unstable (see, for example, https://sharadpra.wordpress.com/2018/03/12/fecl3-stable-but-... ).
[Edited on 14-1-2020 by AJKOER]
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Interesting enough in its way, but utterly irrelevant.
It's not as if anyone was claiming that iodine isn't an oxidant.
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
That's a matter of definition.
There is a solvate
NaI 3 CH3COCH3.
Is it a reaction product? It's not the same as the starting materials.
https://pubs.acs.org/doi/pdfplus/10.1021/ja01417a015
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Interesting to read all the comments. I did have one more go to make calcium iodide in ethanol. This time I first reacted the over-supply of calcium
and ethanol for 90 minutes on reflux without iodine in case the calcium could react with the 5% water. This gave a milky white solution which I let
cool down to room temp (35C) before decanting and vacuum filtering. A very fine white powder remained behind on the filter paper and the filtrate was
clear. Not sure if the white powder is calcium oxide or ethoxide or something else.
I next poured the clear filtrate back with the remaining calcium and added iodine in 2 or 3 g batches under reflux. The iodine of total 9g was quickly
consumed and left a yellow solution after filtering.
Trying to dry this solution and extract something was much more problematic than my previous attempt. It should have been purer that the previous
batch but all I ended up with was a dark very sticky mess that did not want to dissolve in either water or ethanol. For sure the 9g iodine was
included in this mess but in which form or compound I don't know. At no stage was iodine vapour seen, but when it was heated enough a yellow layer
appeared on the sides of the beaker.
I think I'll move onto another compound now!
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
You seem to have chosen a strange path to make iodoform.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4419
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
In the absence of iodine, acetone will react with magnesium (and possibly calcium) to give pinacol. I dare suggest that all you've made is a mess.
Dry ether should work as a solvent, if you've got some.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
2 |