Xrpdguy
Hazard to Self
 
Posts: 51
Registered: 28-2-2018
Location: Serbia
Member Is Offline
Mood: Suspicious
|
|
Microwave inducted synthesis
Hello, there I am very interested in technochemistry especially the microwave.
For every synthesis, I have used a microwave reactor but never a domestic one. Now I am curious about a few things: Why the reaction vessel needs to
be at the center of the rotation plate? and Why should I use 250 ml Erlenmeyer even I have just about 5 ml of the reaction mixture as described in
some scientific paperwork?
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Hey, I am too interested in microwave chemistry, and I think I might answer your first question.
(Disclaimer - this explanation based mostly on my intuition)
First watch this video by nilered(watch from t=9:12 to t=10:39):
https://www.youtube.com/watch?v=l0u8Vtf2GoQ&t=35s
As you can see, microwaves form "hot spots" and "cold spots"(explained in the video).
If the reaction vessel is in the middle of the rotating plate, than the axis of rotation of the vessel will be in the middle of the vessel. This allow
for a more even exposure to any hot/cold spots due to symmetry.
Keep in mind that even when the reaction vessel is in the middle, the exposure still won't be perfectly even. it will merely be better compared to not
putting it in the middle.
Attachment: phpoyTUVg (27kB)
This file has been downloaded 456 times
[Edited on 10-1-2020 by B.D.E]
|
|
|
Xrpdguy
Hazard to Self
 
Posts: 51
Registered: 28-2-2018
Location: Serbia
Member Is Offline
Mood: Suspicious
|
|
I see, but also my area of interest are Ionic liquids. So when I put a vial with BMIM for example in the center of the rotation plate it needs much
more time to get hot than when I place it at the side.
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by Xrpdguy  | | I see, but also my area of interest are Ionic liquids. So when I put a vial with BMIM for example in the center of the rotation plate it needs much
more time to get hot than when I place it at the side. |
It is possible that your microwave produce more "hot-spots" in the peripheral areas rather than in the center.
Putting your vessel in the center merely mean that it would be exposed more evenly to the radiation. It doesn't necessarily mean that it would be
exposed to more radiation.
Maybe there's another explanation for putting the vessel in the center. Maybe it's just more convenient. For instance if the researchers were to check
the repeatability of the experiment, the simplest way of doing it is to always place the vessel in the center of the microwave.
Those are at least my ideas :)
[Edited on 10-1-2020 by B.D.E]
|
|
|
Xrpdguy
Hazard to Self
 
Posts: 51
Registered: 28-2-2018
Location: Serbia
Member Is Offline
Mood: Suspicious
|
|
Quote: Originally posted by B.D.E  | Quote: Originally posted by Xrpdguy  | | I see, but also my area of interest are Ionic liquids. So when I put a vial with BMIM for example in the center of the rotation plate it needs much
more time to get hot than when I place it at the side. |
It is possible that your microwave produce more "hot-spots" in the peripheral areas rather than in the center.
Putting your vessel in the center merely mean that it would be exposed more evenly to the radiation. It doesn't necessarily mean that it would be
exposed to more radiation.
Maybe there's another explanation for putting the vessel in the center. Maybe it's just more convenient. For instance if the researchers were to check
the repeatability of the experiment, the simplest way of doing it is to always place the vessel in the center of the microwave.
Those are at least my ideas 
[Edited on 10-1-2020 by B.D.E] |
More hotspots? I don't think so, its just domestic one (850W max power, mono mode).
In the meanwhile, I made an experiment with a quartz cuvette (because it's totally transparent to microwaves). It took less time (few minutes) to heat
the water when I put a cuvette at the side than to place it in the middle.
Anyway, do you know why should I use the huge vessel for a small volume of reactants? Somewhere on the internet, I found it's because hazardous
explosions, but if something is dangerous to heat in the microwave it will destroy no matter how big vessel is.
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by Xrpdguy  |
More hotspots? I don't think so, its just domestic one (850W max power, mono mode).
In the meanwhile, I made an experiment with a quartz cuvette (because it's totally transparent to microwaves). It took less time (few minutes) to heat
the water when I put a cuvette at the side than to place it in the middle. |
I meant that maybe your microwave produce more hotspots in the sides compared to in the center.
Quote: Originally posted by Xrpdguy  | | Anyway, do you know why should I use the huge vessel for a small volume of reactants? Somewhere on the internet, I found it's because hazardous
explosions, but if something is dangerous to heat in the microwave it will destroy no matter how big vessel is. |
I really don't know.. The only guess I have is to delay pressure build-up inside the vessel in case it's a close system. If you were to heat 15ml in a
close 20ml vial than I can see why it might be a problem. But I don't see a reason to use "huge" vessel..
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
https://www.youtube.com/watch?v=qvpK2Ie1pl0
Microwave inducted synthesis of phthalocyanine blue by U235
"A mind is a terrible thing to lose"-Meisner
|
|
|
Xrpdguy
Hazard to Self
 
Posts: 51
Registered: 28-2-2018
Location: Serbia
Member Is Offline
Mood: Suspicious
|
|
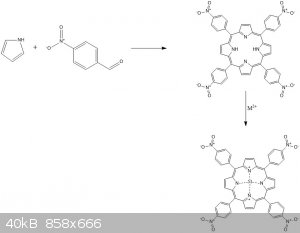
This looks great. A great example of "dry" synthesis, especially because of making macromolecule.
If we talk about dyes my idea was to use pyrrole and p-nitrobenzaldehyde by using nitrobenzene as a solvent to make 5,10,15,20-tetrakis(4-
nitrophenyl)porphyrin and later complex it with some metal. The reaction will be inducted by microwave (about 650W max power).
I am still not sure about the formula of the last compound and how metal is connected with nitrogen atoms. Can anyone help?
[Edited on 12-1-2020 by Xrpdguy]
[Edited on 12-1-2020 by Xrpdguy]
|
|
|
Texium
|
Thread Moved
29-11-2023 at 12:02 |