LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Hands aren’t the same as soft skin!
So, I’ve been slowly venturing back into amateur chemistry in my free time. Was emptying the last bit of 96% sulphuric acid out of a litre bottle
into a 100mL reagent bottle, and managed to spill some. A few mL of it managed to miss the bottle, hit the table, and splash onto me - burned right
through a semi-decent cotton top and shorts, and onto my belly! This is the result 3 days later, it re-emerged after initially disappearing after what
looked like mouth canker sores all over my torso.
Remember, even if your hands are unscathed after half a minute or so of enduring mineral acids, the rest of you probably won’t! This was literally
an immediate response, less than 10 seconds before I applied copious amounts of wet paper towel.
Be careful with your hazardous chemicals, they’re labelled that way for a very good reason. Luckily I only got away with a half an hour or so of
stinging, doing something seemingly so benign.
 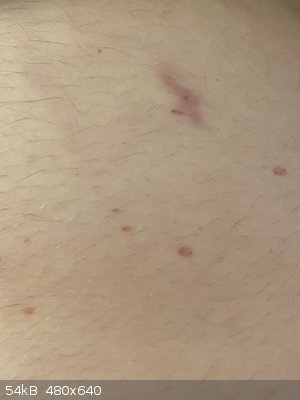
[Edited on 7-1-2020 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by LearnedAmateur  | So, I’ve been slowly venturing back into amateur chemistry in my free time. Was emptying the last bit of 96% sulphuric acid out of a litre bottle
into a 100mL reagent bottle, and managed to spill some. A few mL of it managed to miss the bottle, hit the table, and splash onto me - burned right
through a semi-decent cotton top and shorts, and onto my belly! This is the result 3 days later, it re-emerged after initially disappearing after what
looked like mouth canker sores all over my torso.
Remember, even if your hands are unscathed after half a minute or so of enduring mineral acids, the rest of you probably won’t! This was literally
an immediate response, less than 10 seconds before I applied copious amounts of wet paper towel.
Be careful with your hazardous chemicals, they’re labelled that way for a very good reason. Luckily I only got away with a half an hour or so of
stinging, doing something seemingly so benign.
[Edited on 7-1-2020 by LearnedAmateur] |
Also, from experience - larger amounts of concentrated sulfuric acid on skin should be wiped off with something dry, before washing or wiping with
something wet, exothermic mixing will add a thermal burn to ones mishap.
Otherwise - these things happen, skin is skin and skin will heal, eyes could have ended up way sadder.
|
|
|
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by LearnedAmateur  | | A few mL of it managed to miss the bottle, hit the table, and splash onto me - burned right through a semi-decent cotton top and shorts, and onto my
belly! |
Lucky it went through your cotton top to your belly,
it could have been through your shorts to a more sensitive area 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
Sulfuric is nasty stuff! I always get a feeling that I itch all over when I work with it, although there have been no spills that could have ended up
on my skin.
Exact science is a figment of imagination.......
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
As some one who grew up around batteries and had a metric ton of handling of H2SO4 in all concentrations, you should all ways have several liters of
mild bicarb solution!
Even if you just think a bit got on you wash with bicarb then water, because other wise all you are doing is diluting it, not removing it
for my lab I have 2 4 liter jugs filled with bicarb that is only for use on the body encase of contact, when in eyes seconds count, so put them some
where you can find them even if blinded, and if you remain calm and controlled you will fare fare better then one who panics!
So recap 8L Bicarb solution in a position you can find off memory and feel alone, and practice doing so with eye's closed
If you think some got on you, rinse with bicarb then water.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
That's why I cry and shout and curse when I receive a chemicals in HDPE jugs that will obviously spill drops everywhere when pouring.
Last time I had to empty 5 liters of H2SO4 in two glass bottles I wasnt happy at all ! (but I discovered the real color of the floor)
Out of curiosity, how did you manage so many drop from such a small amount ?
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Because I’m an idiot and treated the stuff like water, I don’t exactly have much in the way of PPE. Honestly, it wasn’t much liquid, probably a
mL or less that ended up on me, it just happened to spatter when it hit the table - the little burns are only 2-3mm in diameter.
[Edited on 30-1-2020 by LearnedAmateur]
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | Quote: Originally posted by LearnedAmateur  | | A few mL of it managed to miss the bottle, hit the table, and splash onto me - burned right through a semi-decent cotton top and shorts, and onto my
belly! |
Lucky it went through your cotton top to your belly,
it could have been through your shorts to a more sensitive area 
|
I know somebody who once got a lap-full of chloroform.
Said it was painful.
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
if you got no lab coat, rain coat it. NileRed did a great video " pouring acid on my my hand". some times it best to not use gloves! but always use
eye protection.
"A mind is a terrible thing to lose"-Meisner
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
That always surprised me had dichloromethane spill on me it was way more painful than HCl acid spill understanding chemistry better the organic
solvents and acids are just plain nastier just take glacial acetic acid it's some nasty corrosive stuff
|
|
|