lordcookies24
Hazard to Self
 
Posts: 78
Registered: 2-1-2019
Location: pluto
Member Is Offline
Mood: curious
|
|
Synthesis of Ammonium Perchlorate
I plan on making Ammonium Perchlorate through the double decomposition reaction between Sodium Perchlorate and Ammonium Nitrate (or any other easy to
find ammonium salts). To make Sodium Perchlorate, most online articles ask perform eletrolysis on hot(70C) aqueous Sodium Chlorate. To make Sodium
Chlorate, I need to perform eletrolysis on hot(70c) aqueous Sodium Chloride. Either i am understanding it completely wrong but since making the
Perchlorate and Chlorate require the excact same condition (eletrolysis of 70°C aqueous solution), shouldn't making Sodium Chlorate make Sodium
Perchlorate as soon as it is produced? I read somewhere else that the best way to make Chlorates is through Pottasium Chloride since they don't
disolve in water. So does Sodium Chlorate directly oxidize to Sodium Perchlorate without an extra step since it disolves in water? And will adding
Ammonium Nitrate to a Sodium Perchlorate solution yield Ammonium Perchlorate? And for the final question are graphite eletrodes (from pencils) fine as
long as I recrystalize the product or do I have to spend 60 bucks on platnium-titanium electrodes?
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
using graphite electrodes you can obtain only NaClO3, not NaClO4
it is possible to electrolytically cover graphite with a layer of PbO2 so then such electrode is capable to produce also ClO4- but Pb is toxic, while
covering the electrode it must be rotating at high rpm (repulsive force >> bubbles adhesive force so the higher diameter of electrode and higher
rpm the better) otherwise bubbles of gas cause defects in layer of PbO2 so fast corrosion when using defective electrode in ClO4- production
when you obtain NaClO3, carefully slowly melting it and heating produces NaCl + NaClO4 with side reaction of decomposition into NaCl+O2 (or total
decomposition into NaCl+O2 without NaClO4  if you overheat it) you can separate
them using acetone (NaCl + NaClO3 insoluble, NaClO4 soluble) if you overheat it) you can separate
them using acetone (NaCl + NaClO3 insoluble, NaClO4 soluble)
do not try to produce K salts as KClO4 has very low solubility so you can't obtain NH4ClO4 from KClO4, you need NaClO4 which is more soluble in water
than NH4ClO4
decades ago it was possible to buy a herbicide Travex in my country which was a mixture of 50:50 NaCl + NaClO3 but it is not produced anymore. It was
sold in 1 kg tight well closed metallic tins and my grandma, God rest her soul, was always surprised how fast was it "leaking" and disappearing from
the can 
Wow some brave soul in my country started something like a private fight against restrictions and found a way how to sell legally NaClO3 to public -
this seller is known to be friendly to young chemists - the shop sells NaClO3 phlegmatized with NaCl so concentration of NaClO3 is below 40% limit,
this mixture is very close to my beloved Travex of my childhood 
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Online
Mood: Volatile
|
|
Quote: Originally posted by Fery  |
Wow some brave soul in my country started something like a private fight against restrictions and found a way how to sell legally NaClO3 to public -
this seller is known to be friendly to young chemists - the shop sells NaClO3 phlegmatized with NaCl so concentration of NaClO3 is below 40% limit,
this mixture is very close to my beloved Travex of my childhood 
|

That's really cool  . I know this seller - I bought from him twice some
chemicals and met him personally in his shop. He is chemist on the first look . I know this seller - I bought from him twice some
chemicals and met him personally in his shop. He is chemist on the first look  . .
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
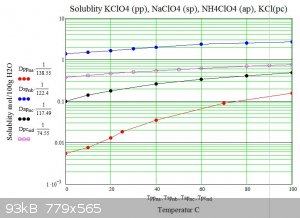
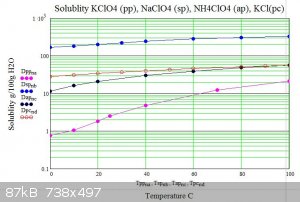
Below are graphs of the solubility of sodium potassium and ammonium perchlorates in water versus temperature. For comparison potassium chloride is
also shown.
The graphs show how potassium perchlorate has approximatly 200 times less solubility than sodium perchlorate at 0C. .
The usual double decomposition synthesis requires that the target salt is insoluble or has the lowest solubility of the reactants and products of
the decomposition at some temperature. As can be seen from the graphs ammonium perchlorate has three times the solubility of potassium perchlorate at
any temperature hence water solutions can not be used to produce ammonium perchlorate from potassium perchlorate in the usual way.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If you can make sodium perchlorate, then it is easier to make ammonium perchlorate through HClO4. In order to do so, boil down the solution of NaClO4
(no need to really separate it) and add concentrated HCl (36% or so) in excess amount. This produces a lot of precipitate of NaCl and leaves HClO4 in
solution. Any NaClO3 is destroyed in this way and also any NaCl is precipitated. What remains is a mixed solution of HClO4 and HCl, which also
contains a little NaClO4. Boil down this liquid to appr. 150 C and then allow to cool. A little more NaCl may precipitate.
To this liquid add ammonia until the liquid smells of ammonia (you must have a little excess ammonia, otherwise you get a very hygroscopic product,
which is hard to crystallize). Then allow the water to evaporate at a warm place. Do not let it evaporate to dryness, once you get a lot of crystals,
covered in a thin layer of liquid, then decant the liquid and dry in filter paper. In this way you get fairly pure NH4ClO4 with only little sodium
left in the product.
|
|
|
jderimig
Harmless

Posts: 10
Registered: 25-11-2019
Member Is Offline
|
|
If you have sodium perchlorate its even easier to add ammonium chloride (cheaper) to the solution. Cool and ammonium perchlorate will crystallize
out.
The hard part is getting the sodium perchlorate. Also you must kill and sodium chlorate before the ion exchange reaction or you produce traces
amounts of ammonium chlorate which extremely explosive and unstable.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Indeed, the hard part is getting NaClO4. But once you have that, the method through HCl is easier than the method through NH4Cl, even though it has
more steps. The method through conc. HCl removes much more sodium ions and it destroys any chlorate, present in your NaClO4.
|
|
|
jderimig
Harmless

Posts: 10
Registered: 25-11-2019
Member Is Offline
|
|
In your process what happens to the excess HCL?
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If you boil down the liquid to nearly 150 C then you drive off the HCl, while HClO4 remains behind (the water/HClO4 azeotrope boils at 220 C or so,
while the water/HCl azeotrope boils below 120 C).
|
|
|
lordcookies24
Hazard to Self
 
Posts: 78
Registered: 2-1-2019
Location: pluto
Member Is Offline
Mood: curious
|
|
Thank you for all the comments guys, I have a lot more to go on. I guess buying Mixed Metal Oxide electrodes will be the way to go for this
electrolysis.
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Does hypochlorite react with ammonium nitrate
Does it make hydrazine nitrate
I know it reacts with ammonia and urea
|
|
|