Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Dehydrogenation of Diels Alder addition products
While looking into the chemistry of potassium sorbate I came across some paper that describe the Diels Alder type condensation of sorbic acid with
maleic anhydride (1) for instance to yield the partial anhydride of 4-methyl-2,5-cyclohexadiene 1,2,3-tricarboxylic acid, this apparently tends to
decarboxylate to give 2,3-anhydride. If this could be dehydrogenated to an aromatic compound it would give 3-methylphthalic anhydride.
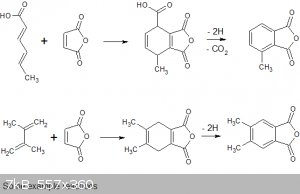
Similarly 2,3-dimethyl-1,3-butadiene (available from pinacol) condenses to give 4,5-dimethyl-1,4-cyclohexadiene-1,2-dicarboxylic acid which on
dehydrogenation would give 4,5-dimethylphthalic anhydride. The condensations are well established but I can't find any examples of the dehydrogenation
or oxidation of the compounds to phthalic acids.
Does any one have any ideas as to the sort of reagents might bring this about. Sulphur at high temperatures was once used but the conditions are a bit
brutal. Would say, Pd on charcoal under vacuum, work (reverse hydrogenation)? Or maybe an oxidizing agent such as iodine?
I did find a few Indian papers that reported phthalic acid derivatives but they used acetylenic derivatives and aryl substituted crotonate
derivatives.
1) Craig and Shipman; JACS, v74; is5; p2905, [1952]
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
The chemistry of sorbic acid is a long standing question in my mind too!  It is a
fairly cheap and OTC available diene and it would be interesting to make something useful/unusual/pleasant smelling compound from it. It is a
fairly cheap and OTC available diene and it would be interesting to make something useful/unusual/pleasant smelling compound from it. 
As for the dehydrogenation my first bet 'd have been sulfur. I read sometime somewhere that dehydrogenations with sulfur are not always too brute
force, depending on the substrate - and your definition on brutality.
My second (similarly old-school) bet 'd have been distillation from zinc powder. (Although with an anhydride it may not be usable, but as I remember
it was used e.g. in the structure determination of koniine. Distillation of koniine (2-propyl-piperidine) gave 2-propil-pyridine and its oxidation
product 2-picolinic acid was a known compound.)
Your suggested "reverse hydrogenation" may also be a good method and is surely less stressful to the substrate - unless there's an unexpected quirk.
Which is so many times the case in organic chemistry. 
|
|
|
Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Pumukli, yes its surprising that there is not a single thread on SM dedicated to sorbates or sorbic acid chemistry in spite of it being one of the
most accessible dienes and very cheap. I have done a fair bit of chemistry on sorbic acid, I have prepared the 4,5-dibromo and 2,3,4,5-tetrabromo
addition products. I tried reacting these with thiourea and then treating the product with alkali interestingly you appear to get a thiazolidine
derivative rather than a dithiol. I have collected a whole series of papers regarding the reaction of nitrites with sorbic acid. They are mostly
investigations into the food safety of potassium sorbate as a food additive and the experiments were caried out at high dilution but it would be
interesting to see what results at preparative concentrations.
The tetrabromo addition product forms hard glassy colourless crystals of 2,3,4,5-tetrabromohexanoic acid. I also managed to reduce sorbic acid to
3-hexenoic acid which can then be brominated to 3,4-dibromohexanoic acid. So there is a lot of scope to prepare interesting derivatives.
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
I collected some sorbic acid papers during the past years. I was mostly interested in the Diels-Alder reaction so I probably have the same articles
what you have too. 
Even making a simple ester of this acid is a challenge because as I remember the diene is sensitive to acid so a Fischer esterification won't work.
(But I may be entirely wrong regarding the reaction, it was long ago when I studied this compound.)
That 3-hexenoic acid is an interesting compound though. I'd like to read about your adventures with its synthesis and all! 
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Quote: Originally posted by Boffis  | While looking into the chemistry of potassium sorbate I came across some paper that describe the Diels Alder type condensation of sorbic acid with
maleic anhydride (1) for instance to yield the partial anhydride of 4-methyl-2,5-cyclohexadiene 1,2,3-tricarboxylic acid, this apparently tends to
decarboxylate to give 2,3-anhydride. If this could be dehydrogenated to an aromatic compound it would give 3-methylphthalic anhydride.
1) Craig and Shipman; JACS, v74; is5; p2905, [1952] |
Hi Boffis, according the dehydrogenation using sulfur I saw it in abietic acid -> retene without brutal conditions (IIRC it was just heating
together at atmospheric pressure). Abietic acid is present in conifer rosin = colofony from which it could be isolated (isomerization +
crystallization) which I did decades ago. Rosin is a protection of plants against diseases but seems to be plant specific and no usage in human
medicine, the same as betulol from birch bark which was investigated but no significant usage in human medicine yet.
I found even older experiment (from 1947) where they compared big scale sorbic acid + maleic anhydride (cca 500g) when the exothermic reaction heats
itself to 240-250 C and the decarboxylation occurs vs. controlled (small scale cca 10 g, or controlled incremental addition at 100g scale when T kept
at 130 C so without decarboxylation or performing the reaction in benzene again without decarboxylation).
Attachment: wicks1947.pdf (313kB)
This file has been downloaded 760 times
Could you post your method of preparing 3-hexenoic acid from sorbic acid please?
|
|
|
Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Fery, Thanks for the info, I have seen some of these papers before, though not the Wicks one but I have several other on essentially the same
reaction (Like my ref. 1 above). Both products are of interest to me if I can make them aromatic, I might give it a go and dehydrogenate with sulphur
or possible Ni catalyst and vacuum like the prep for bipyridyl.
I am also interested in the condensation of benzoquinone with sorbic acid, I don't know if you have come across any refs. for this reaction but I
would be intereste in them if you have.
I have typed up my notes from my experiment on the reduction of sorbic acid with dithionite and willpost them on the sorbic acid thread shortly.
http://www.sciencemadness.org/talk/viewthread.php?tid=155475
[Edited on 3-12-2020 by Boffis]
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Boffis, cool! I have dithionite at hand and also sorbic acid. Looking forward inspirations what to do. Also hydroquinone in stock which could be
easily oxidized to benzoquinone using MnO2 https://prepchem.com/synthesis-of-p-benzoquinone/
Also naphtoquinone could be interesting https://www.prepchem.com/synthesis-of-1-4-naphthoquinone/
|
|
|