Smoker Root
Harmless

Posts: 35
Registered: 29-7-2019
Member Is Offline
|
|
pH measurement tool?
Hi, I am going to procced on a reaction which is extreamly pH sensitive. I am working with materials that corrode easily plastics and needs a pH
measuring tool which can preciley measure in the range of 5.0-8.0
I can't find any of this precision in a normal pH paper, and the elctronic pH readers I have looked uo, all have plastic.
So what should I get?
Thanks
|
|
|
Sulaiman
International Hazard
    
Posts: 3727
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
What do you consider to be precise ?
(+/- 0.1 pH, +/- 0.01pH etc.)
I bought two cheap digital +/-0.1pH resolution meters,
probably my most useless chemistry hobby purchase 
I now use pH papers for approximate measurements (so far good enough for my purposes)
and VERY occasionally, titration for accurate measurement of molarity, which could be converted to pH.
eBay has pH papers for many ranges
(1-14, 3.8-5.4, 5.4-7.0, 6.4-8.0, 6.9-8.4, ....)
if you need better accuracy than that then I guess that you are looking at
a very expensive pH meter with buffered pH reference solutions and a lot of care,
or titrations.
from memory, pH measurment is quite temperature sensitive in the range that you are considering.
EDIT: copied this graph from Researchgate https://www.researchgate.net/figure/Variation-of-pH-vs-tempe...
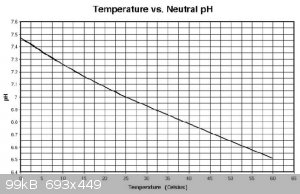
[Edited on 14-10-2019 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
The glass bulb itself in a pH sensor is all glass I believe. And you can get some pretty long ones (30 cm certainly, perhaps even longer), with the
plastics (connector etc) at the other end. Perhaps you can shield the connector end from your reaction mixure?
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
For pH testing, the cheap chinese pH meters from Ali are adequate. As a trick, after you do a reading, immediately dip the probe in cold, distilled
water to neutralize your chem. Or rinse it in cold running water. Do two or three readings in a row, and compare (and average) the results.
Using pH calibration solutions, I find that they are fairly accurate, and I prefer them to pH paper. I have two of them and have used them to test the
pH of beer wort and yeast solutions. It does the job, and at reasonable pH levels, they remain accurate for a long time! 
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
G-Coupled
Hazard to Others
  
Posts: 287
Registered: 9-3-2017
Member Is Offline
Mood: Slightly triturated
|
|
To what kind of values of 'reasonable'? Just not at either of the far extremes?
I have also seen some more glass bulb pH meters that have the bulb at the end of a (presumably also glass) stick.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
They are likely to be plus or minus 0.1 pH unit. Also make sure the probe is stored wet and close to pH neutral.
|
|
|
G-Coupled
Hazard to Others
  
Posts: 287
Registered: 9-3-2017
Member Is Offline
Mood: Slightly triturated
|
|
Quote: Originally posted by B(a)P  | | They are likely to be plus or minus 0.1 pH unit. Also make sure the probe is stored wet and close to pH neutral. |
Any good ideas for a DIY solution to store probes in?
|
|
|
Carbon8
Harmless

Posts: 34
Registered: 1-1-2018
Member Is Offline
Mood: No Mood
|
|
Cole-Parmer suggests 4M KCl for the conditioning and storage of electrodes.
https://www.coleparmer.com/tech-article/ph-electrode-care
|
|
|
G-Coupled
Hazard to Others
  
Posts: 287
Registered: 9-3-2017
Member Is Offline
Mood: Slightly triturated
|
|
Cheers!
|
|
|