| Pages:
1
2
3
..
19 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Synthesis of longer chain tertiary alcohols
1. Introduction:
I’m opening this thread with permission and on behalf of Nicodem and on request by me.
For those still unfamiliar with the currently hottest topic in the ‘Chemistry in General’ section, the small scale production of potassium
metal by non-electrochemical means, I would suggest to read this thread here:
http://www.sciencemadness.org/talk/viewthread.php?tid=14970
And in particular woelen’s (moderator of the ‘Chemistry in general’ section) excellent ‘executive summary’ which sums up current state of
the art of this highly interesting and useful chemical reaction:
http://woelen.homescience.net/science/chem/exps/synthesis_K/...
The overall reaction is KOH(s) + Mg(s) === > K(l) + MgO(s) + ½ H2(g) @ about 200C, in an inert paraffinic/naphtenic solvent. Equipment? A pyrex
flask and a refluxer, essentially…
The natural kinetic hindrance to this reaction is overcome by means of a catalyst, namely t-butanol (2-methyl-2-propanol) which reacts with KOH in the
given circumstances:
t-BuOH + KOH === > t-BuOK + H2O
The ‘miracle’ of potassium production is then achieved by a previously unknown or little known redox reaction:
2 t-BuOK(dissolved) + Mg(s) === > (t-BuO)2Mg(dissolved?) + 2 K(l)
In the mean time successful production of potassium using 2-methyl-2-butanol (t-amyl alcohol or t-pentanol) has also been achieved…
2. Production of sodium:
It rather follows logically that if potassium can be produced in this way, possibly other alkali metals can too. And indeed the US patent on which our
success was based does also give an example of the production of sodium metal using the same principle: reduction of a sodium alkoxide with solid
magnesium in an inert solvent at about 200C. Unfortunately the method prescribes inordinately long ‘cooking’ times of up to 13 hours, hardly
practical for the home chemist.
Among those who’ve been most proactive in bringing about successful replication of the ‘potassium patent’ it’s widely believed that reduced
solubility of the equivalent sodium alkoxides in paraffinic/naphtenic solvents, possibly due to the higher lattice energy associated with the smaller
Na+ ion (compared to K+), is the cause of the slower reaction rate.
If so, the solution to reduce reaction times in the case of sodium would possibly be the use of longer chain tertiary alcohols. Of potential interest
here would be:
• 2-methyl-2-pentanol
• 2-propyl-2-butanol
• 2-ethyl-2-pentanol
• 2-propyl-2-pentanol and such like
• ‘tertiary fatty alcohols’
An interest has thus arisen in the synthesis of such alcohols with a very practical purpose.
Nicodem has kindly pledged (time allowing) to put up a review of the synthesis of tertiary alcohols and I would like to appeal to all organic wiz kids
in this section to contribute with whatever relevant knowledge on the subject that you might have. May this become a very sticky thread!
Thank you.
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
I've successfully synthesized 3-methyl-3-pentanol from ethyl magnesium bromide and ethyl acetate. Both precursor chemicals are easy to obtain otc.
I'm going to quote my procedure from a previous post of mine which can be found in this thread.
http://www.sciencemadness.org/talk/viewthread.php?tid=13844#...
Quote: Originally posted by rrkss  | Well I am done with the grignard. It was my first grignard reaction and was very interesting. I started it by adding 1 mL of ethyl bromide to 60 mL
of ether in a 250 mL roundbottom with 13g of magnesium. I calculated a 0.7g excess of magnesium from what I needed.
The reaction took off and was very exothermic forcing me to put the flask in an icebath right away since my condenser was not catching all the boiling
ether. Once things calmed down I added the ethyl bromide dropwise over the next 2 hours. The grignard reagent was a nice dark gray almost purplish
color.
After the grignard was prepared, I added the ethyl acetate dropwise. The ethyl acetate reacting with the grignard was even more exothermic than the
preparation of the grignard and took a ton of work to get the addition rate just right.
Once the reaction was done, I quenched with acid. I added the 2.0 M H2SO4 solution dropwise to the flask. The addition was highly exothermic and
formed a white jellylike precipitate. I soon ran out of room and was forced to transfer this mess into a larger flask and do multiple acid rinses.
Being strapped for time I ended up probably using way to much acid to quench the reaction (500 mL of 2 M H2SO4 solution) but eventually all the
magnesium reacted and the precipitate dissolved. I then transferred my organic layer to a new roundbottom and dried it with anhydrous MgSO4.
I lost about 50 mL of organic layer during the workup. Probably due to ether's slight solubility in water and my hastly workup. Hopefully after
distillation, did not lose too much of my alcohol which supposedly is slightly soluble in water as well. Tommorow when I distill the ether away and
get my product, I will finally know.
Things I learned.
1. Transfer the finished reaction mixture to a different and larger flask before doing the acid quench.
2. Do the quench when you are not strapped for time as it takes much longer than expected.
|
The alcohol decomposes upon distillation so it will need to be purified under reduced pressure.
[Edited on 12-29-10 by rrkss]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks!
Quote: Originally posted by rrkss  | I've successfully synthesized 3-methyl-3-pentanol from ethyl magnesium bromide and ethyl acetate. Both precursor chemicals are easy to obtain otc.
The alcohol decomposes upon distillation so it will need to be purified under reduced pressure.
[Edited on 12-29-10 by rrkss] |
Does this mean that 3-methyl-3-pentanol would not survive the 200C needed for our alkali alkoxide reductions?
I seem to understand the general reaction path, so let me see if I can come up with some creative (but not necessarily possible!) molecule
rearranging. Bit rusty on the old organic side, you see 
According to that scheme, 2-ethyl-2-pentanol (for instance) could be synthesized from methyl propanoate and propyl magnesium bromide...
[Edited on 29-12-2010 by blogfast25]
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
AFAIK, you have to wash the ether layer with some aqeous sodium carbonate solution, as traces of acid decompose the alcohol by a elimination reaction
(E1 mechanism).
During heating I think it just decomposed, giving you water and a alkene.
|
|
|
NurdRage
Hazard to Others
  
Posts: 182
Registered: 11-11-2010
Member Is Offline
Mood: No Mood
|
|
I'm doing a bit of long-chain t-alcohol synthesis on the side. Currently i'm starting with 1-octadecene and trying to couple that with acetone (with
several steps in between) to get a C21 tertiary alcohol.
As for amateur approaches, a problem i see is finding suitable domestically available long-chains to start with. The most readily available activated
long-chain carbon sources i know are 2-butanone, ethyl acetate, acetone, ethanol and methanol.
Any others that i missed?
[Edited on 29-12-2010 by NurdRage]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by NurdRage  | I'm doing a bit of long-chain t-alcohol synthesis on the side. Currently i'm starting with 1-octadecene and trying to couple that with acetone (with
several steps in between) to get a C21 tertiary alcohol.
As for amateur approaches, a problem i see is finding suitable domestically available long-chains to start with. The most readily available activated
long-chain carbon sources i know are 2-butanone, ethyl acetate, acetone, ethanol and methanol.
Any others that i missed?
[Edited on 29-12-2010 by NurdRage] |
The esters are relatively easy to synthesise though, are they not?
[Edited on 29-12-2010 by blogfast25]
|
|
|
NurdRage
Hazard to Others
  
Posts: 182
Registered: 11-11-2010
Member Is Offline
Mood: No Mood
|
|
In a grignard, the alcohol side of the ester is lost.
Nonetheless they play a "chain doubler" role in that they add two grignard reagents to the acid side. so you get C2n+m where n is grignard carbon
length and m is the carboxylic acid carbon length.
As carbon sources themselves we'd need a long-chain carboxylic acid, i don't know of any other than acetic acid.
I suppose though with esters the problem simplifies to that if we can get a good long chain alcohol we could convert it to a grignard and pull a
chain-doubler trick to essentially get twice the chain length (plus acid length) we started with.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
The decomposition is dehydration to yield the olefin. This is especially pronounced for the aforementioned tertiary alcohol. 2° and 1° alcohols
will not be as easy to dehydrate, but in the presence of sulfuric acid, particularly if you are concentrating it while the distillation takes place,
most will react (at least to some extent, at the expense of yield).
The aqueous solubility of the product is poor (45 g/L), so I would recommend salting-out the aqueous phase and extracting it into a more volatile
organic solvent.
The BP is 122.4 °C, BTW.
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Does this mean that 3-methyl-3-pentanol would not survive the 200C needed for our alkali alkoxide reductions?
[Edited on 29-12-2010 by blogfast25] |
It should survive. The decomposition was more charring on the glass but it did distill over. This alcohol has a high boiling point so there was lots
of localized heating causing the charring. When I distilled under vacuum the charring problem was eliminated.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by NurdRage  | In a grignard, the alcohol side of the ester is lost.
Nonetheless they play a "chain doubler" role in that they add two grignard reagents to the acid side. so you get C2n+m where n is grignard carbon
length and m is the carboxylic acid carbon length.
As carbon sources themselves we'd need a long-chain carboxylic acid, i don't know of any other than acetic acid.
I suppose though with esters the problem simplifies to that if we can get a good long chain alcohol we could convert it to a grignard and pull a
chain-doubler trick to essentially get twice the chain length (plus acid length) we started with.
|
What about butyric acid? Should be quite easy to make... And the other, longer fatty acids? Nicodem mentioned something about margerine...
[Edited on 29-12-2010 by blogfast25]
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|
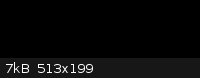
Octadecanoic acid (stearic acid) from candles would be easy enough to estrify with e.g. methanol and further react that FAME (Fatty Acid Methyl Ester)
with ethyl magnesium bromide yielding 1,1-diethyloctadecan-1-ol if I understand rrkss's reaction scheme right and NurdRage's explanation correct.
Please correct me if i am wrong.
Pic of suggested compound:
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bahamuth  | Octadecanoic acid (stearic acid) from candles would be easy enough to estrify with e.g. methanol and further react that FAME (Fatty Acid Methyl Ester)
with ethyl magnesium bromide yielding 1,1-diethyloctadecan-1-ol if I understand rrkss's reaction scheme right and NurdRage's explanation correct.
Please correct me if i am wrong.
Pic of suggested compound:
|
Actually going by the picture and assuming you’ve got the reaction right (I’m pretty lost for the moment! Must read up on Grignards…) you’ve
got 3-ethyl-3-icosanol there: the longest chain is 20, not 18. But that’s definitely a long chain tertiary alcohol!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
This was Nicodem's remark over at the K thread with regards to Grignarding margerine...
Quote: Originally posted by Nicodem  | IUsing a longer chain t-alcohol as catalyst might prove useful in the synthesis of sodium where t-BuOH is unlikely to work given the practical
insolubility of t-BuONa in alkanes. A crude way to "fatty t-alcohols" can be the reaction of a large excess of MeMgI or EtMgI(Br) with pure margarine
in refluxing THF or other ethers. The preparation of grignard reagents require Mg and dry solvents. Purifying the products would require some
knowledge of organic chemistry, but already with extractions it might be possible to get them pure enough for this reaction. I doubt it requires a
very pure catalyst, as all the impurities would be destroyed by the reaction conditions (hot KOH!).
|
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Oh, I get it now. Ester + 2 RMgX causes the alcohol of the ester to split off and then the second R group is added on and the =O converted to –OH.
If R = methyl you get a 2-methyl-2-… ol, with R = ethyl a 3-ethyl-3-… ol, with R = propyl a 4-propyl-4-… ol. All tertiary (and all ‘in
theory’!)
Caproic (hexanoic) acid esters must be quite OTC and with methyl magnesium halides would give 2-methyl-2-octanol. Already quite a long chain really…
Methyl butanoate is a food additive.
[Edited on 29-12-2010 by blogfast25]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by NurdRage  | | As carbon sources themselves we'd need a long-chain carboxylic acid, i don't know of any other than acetic acid. |
Alkali salts of propanoic acid (CH3CH2COOH) are readily available as food preservatives, where they are more commonly known as
propionates.
|
|
|
bahamuth
Hazard to Others
  
Posts: 384
Registered: 3-11-2009
Location: Norway
Member Is Offline
Mood: Under stimulated
|
|
Quote: Originally posted by blogfast25  | Quote: Originally posted by bahamuth  | Octadecanoic acid (stearic acid) from candles would be easy enough to estrify with e.g. methanol and further react that FAME (Fatty Acid Methyl Ester)
with ethyl magnesium bromide yielding 1,1-diethyloctadecan-1-ol if I understand rrkss's reaction scheme right and NurdRage's explanation correct.
Please correct me if i am wrong.
Pic of suggested compound:
|
Actually going by the picture and assuming you’ve got the reaction right (I’m pretty lost for the moment! Must read up on Grignards…) you’ve
got 3-ethyl-3-icosanol there: the longest chain is 20, not 18. But that’s definitely a long chain tertiary alcohol!
|
You are indeed correct. Never heard of or used the alkane name Icosanol before though.
According to this: http://chemistry2.csudh.edu/rpendarvis/carb-enolate.html#est... the suggested product of stearic acid and EtMgBr would be 3-ethyl-3-icosanol by
reaction:
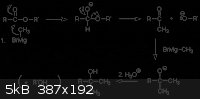
High boiling point would be expected, perhaps over 250 degrees C, and "fatty" enough, a bit bulky though.
Stearic acid is OTC for hand lotion and candle hobby production everywhere in pure enough form.
Quote: Originally posted by blogfast25  | Oh, I get it now. Ester + 2 RMgX causes the alcohol of the ester to split off and then the second R group is added on and the =O converted to –OH.
If R = methyl you get a 2-methyl-2-… ol, with R = ethyl a 3-ethyl-3-… ol, with R = propyl a 4-propyl-4-… ol. All tertiary (and all ‘in
theory’!)
Caproic (hexanoic) acid esters must be quite OTC and with methyl magnesium halides would give 2-methyl-2-octanol. Already quite a long chain really…
Methyl butanoate is a food additive.
[Edited on 29-12-2010 by blogfast25] |
In theory yes, not all who struggle to get sodium metal can get their hand on or synthesize Grignard reagents and/or get hold of suitable solvents.
Anyways, I was intrigued by the synthesis as I have yet to do a Grignard at all.
[Edited on 29-12-2010 by bahamuth]
Any sufficiently advanced technology is indistinguishable from magic.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks bahamuth! The name for the C20 n-alkane is icosane, its alcohols would be icosanols...
Methyl hexanoate 1 kg from sigma-aldrich for £42:
http://www.sigmaaldrich.com/catalog/ProductDetail.do?D7=0&am...
Sample (unspecified size) £15.
[Edited on 29-12-2010 by blogfast25]
[Edited on 29-12-2010 by blogfast25]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by NurdRage  | | As carbon sources themselves we'd need a long-chain carboxylic acid, i don't know of any other than acetic acid. |
Grignard reagents can also be used to build up carboxylic acids by treatment with CO2 as dry ice:
R-MgX + CO2 --> --> R-COOH
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
If you want stearic acid, saponify lard (manteca). You can't get much cheaper than that. Palm oil triacylglyceride is primarly n-hexadecanoic
(palmitic).
A mixture of C20-C30 n-alkanols is known a policosanol. This is frequently derived from sugar cane cuticle wax. An excellent substitute for carnauba
wax, it shares similar issues with solubility. n-C28OH, octacosanol (the dietary suppliment), for example, is only very sparingly soluble in DCM, or
better, chloroform...and not much else (or so it appears to me, having isolated it from sugarcane process mud).
They are quite crystalline and difficult to re-dissolve once isolated.
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
NurdRage
Hazard to Others
  
Posts: 182
Registered: 11-11-2010
Member Is Offline
Mood: No Mood
|
|
Yeah.... if we go TOO long it won't be soluble... which kinda defeats the purpose of making this in the first place; to make sodium ions soluble in
aliphatic solvents.
coming in from a completely different direction: could we make crown ethers by amateur means? t-butanol and a crown ether (say 15-crown-5) might do
the trick and solvate sodium ions... assuming the ether remains stable under these harsh conditions.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Personally I very much like the idea of a 2-methyl-2-alkanol with total chain length around C10. These could be Grignarded with
MeMgBr on linear long chain alkanoates, with ‘alka ≈ C10’. They would have the same ‘active site’ (2-methyl-2ol) as t-butanol but a
medium-long paraffinic tail for solubility in alkanes/napthenics.
Fairly OTC precursors appear to be:
Ethyl octanoate: C2H5OOCC7H15, food additive (pineapple, apple like aroma).
Decanoic acid: C9H17COOH, used in perfumery, lubricants, greases, rubber, dyes, plastics, food additives and pharmaceuticals (Wiki).
Methyl nonyl ketone: CH3COC9H19, used in pest control, as cat repellant.
It’s hard to believe that, say sodium 2-methyl-2-octanoate, would not be quite soluble in Shellsol or equivalent.
Much longer chain alkoxides would need much higher catalyst/hydroxide ratios. For KOH the ratio is currently roughly 0.1 ml of t-butanol / 1 g
of KOH) (for NaOH that would already be higher to account for the lower MW of NaOH). For a t-alcohol with twice the chain length as t-butanol that
ratio would approx. double and for, say C20, it would have to be multiplied by about 5! The catalyst cost then becomes a real factor… And these
octyl-nonyl-decyl precursors might not come very cheap!
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Perhaps. If the solubility increases and subsequently transfer mobility does likewise, you'll certainly
need less catalyst per mole. You might also need less per gram.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@Watson:
Probably. The reaction rate in the case of a successful 2-methyl-2-alkanol will depend mainly on:
• Volatility: as pointed out by you early on in the K thread, volatile alcohols will concentrate more in the vapour phase. Higher boiling t-alcohols
have the edge there.
• The equilibrium constants of the initiation, propagation and termination steps are all likely to be a function of n, the length of the alkane
backbone.
I wonder also if it would be worth revisiting primary or secondary alcohols but long chain ones: octanol, nonanol or decanol for instance. So
far no one has explained why the alcohol has to be a tertiary one and it is possible the patent authors chose t-butanol because at least it’s
slightly less volatile than short chain primary alcohols.
Or how about 2-methyl-2-ols derived from the naphthenic acids 'isomer' mix:
http://en.wikipedia.org/wiki/Naphthenic_acid
[Edited on 31-12-2010 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Some sources of potentially interesting precursors:
Berje (US):
Methyl heptylate (?? Methyl heptanoate ??)
Methyl-2-nonenoate (??)
Methyl nonyl ketone
Nonenal-cis-6
Nonenol-cis-6
3-octanol
Web: http://www.berjeinc.com/chemmp.html
Oxford Chemicals (UK):
Methyl octanoate
http://www.oxfordchemicals.com/oxford/ocweb.nsf/0/18510EF22B...
Grau aromatics (Germ.):
Capric acid (decanoic acid)
Caproic acid (hexanoic acid)
Capylic acid (octanoic acid)
Heptanol
Valeric acid (pentanoic acid)
Lauryl alcohol (dodecanol)
Myristic acid (tetradecanoic acid)
Octanol
Pelargonic acid (nonanoic acid)
http://www.grau-aromatics.de/fileadmin/dokumente/flavourfrag...
Penta group:
Probably the largest inventory, includes 1-octanol, 2-octanol, 3-octanol
http://www.pentamfg.com/query.asp?page=193
SAFC global:
Methyl octanoate 99 %
http://www.safcglobal.com/catalog/ProductDetail.do?N4=W27280...
Ungerer (global):
ethyl hexanoate, octanoate, decanoate
http://www.ungererandcompany.com/content/view/23/33/
Source page:
http://www.thegoodscentscompany.com/data/rw1008791.html
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Or isopropyl myristate – isopropyl tetradecanoate: the non-aqueous component of the two-phase mouthwash, Dentyl pH.
Ready for double Grignarding with CH3MgBr to 2-methyl-2-pentadecanol? It doesn’t get more OTC than that!
[Edited on 31-12-2010 by blogfast25]
|
|
|
| Pages:
1
2
3
..
19 |