FormerBeagle
Harmless

Posts: 8
Registered: 7-6-2019
Member Is Offline
|
|
Tips on Hg manometer?
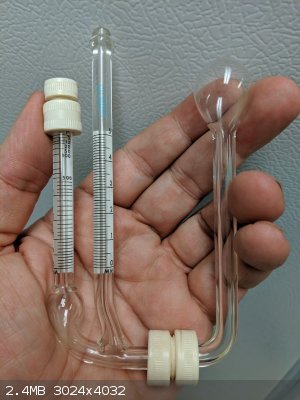
Can anyone give tips on operation/filling of this vacuum manometer? The right hand scale shows 0-5 mm, and the left tube has a scale with a low
number of 2 microns at the top.
Does the bulb stay upward during operation? If all 3 tubes are full, won’t Hg get sucked into the vac line? How is it read? I’ve only used a
U-tube type manometer before.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Does this help?
https://www.sigmaaldrich.com/catalog/product/aldrich/z407496...
Anyeway, the mercury isn't pulled into the pump because there's no air in the system to push it.
I found some instructions for a different model.
They might help.
https://www.aceglass.com/dpro/attachment_files.php?s=80ccd03...
|
|
|
FormerBeagle
Harmless

Posts: 8
Registered: 7-6-2019
Member Is Offline
|
|
That helps hugely, thanks! So it is called a swivel McCleod guage. I think I knew that at one point. Looks like those instructions should get me
started. Unsure when to read the linear and when to read the log scale, but maybe that will become apparent when I read more closely and put it into
service.
And close to $500 from Aldrich!?!
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
You use the non-linear scale fro very low pressures.
Mind you, these things aren't great for chemistry lab use, because there's usually condensable stuff in chemistry systems.
They are useful for checking other manometers/ pressure gauges with.
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
It a McLeod type of gauge. The mercury is used to compress a sample of the vacuum in the capillary tube on the left when the tube with the bulb on the
right is raised.
The difference in height between the top of the mercury in the capillary tube and the top of the mercury in the center tube generates the pressure to
compress the gas in the capillary.
In order to determine the vacuum pressure from the heights of the two mercury column you need to know the compression ratio which is determined by
the change in volume as the mercury fills the left hand capillary tube, note the bulb at the bottom of the left hand capillary tube. The scales on the
left hand capillary tube appears to take into account the compression ratio.
Wiki has an article on this type of gauge.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Texium
|
Thread Moved
29-11-2023 at 11:52 |