| Pages:
1
2 |
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Reducing sodium molybdate by nascent hydrogen
Hi.
I did the experiment with sodium molybdate with nascent hydrogen today. I prepared solution of sodium molybdate and added few mils in to the test
tube. Than I added cca the same portion of concentrated HCl. After that I added a very small piece of aluminum foil and cover test tube with paper
tissue. After half a minute aluminum foil was start vigorously reacting with HCl and hydrogen was start to releasing. On the surface of the foil was
start appearing some yellow compound which was dissolving in water. Solution became yellow and after minute it turned in orange. Before reaction I
suspected that sodium molybdate will be reduced to MoO2 or metallic Mo. But I surprised this colour changing. This isn't metallic Mo and MoO2 is
purple and insoluble in water. This orange compound didn't visibly react with NaOH and Na2S2O5 but did react with KMnO4 to form colourless solution (I
suspected that is molybdate(VI)).
I've done some research in book and on the internet. I've found in book that exists coloured compounds of Mo and W called molybdenum (or tungsten)
bronzes which formed by reduction by hydrogen, molybdenum (or tungsten) or by electrochemical reduction. About this compound is also article on wiki.
https://en.wikipedia.org/wiki/Molybdenum_bronze
About molybdenum compounds is something there:
https://books.google.cz/books?id=Bkr-BAAAQBAJ&pg=PA723&lpg=PA723&dq=molybdenum(IV)+oxide+preparation&source=bl&ots=LyR1rbWyIf&
sig=ACfU3U1X3qnRh9Z4xJqRrilY8ad_ksrCLA&hl=cs&sa=X&ved=2ahUKEwjJ9L_2qsbiAhUJEVAKHZvJBjo4FBDoATABegQICBAB#v=onepage&q=molybdenum(IV)%20o
xide%20preparation&f=false
There is mention about Mo(III) solution which is red. Mo(OH)3 is prepare by action of some metals on molybdate(VI) solution. This compound is soluble
in HCl. There is also something about molybdates(IV) - especcialy about baryum and strontium molybdates(IV) which is also in shades of red. They are
make by reduction of Sr or Ba molybdate(VI) by hydrogen. Maybe I'll try my reaction with addition of some strontium nitrate.
So what do you mean?
[Edited on 1-6-2019 by Bedlasky]
   
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
This compound is more interesting than I thought. I did few tests today and I'll do more test in future. I also will do manganometry analysis of it -
I'll calculate average oxidation number of Mo in this compound.
Here are my observations:
Na2MoO4 + Al (or Zn) + conc. HCl --> orange Mo (+ conc. HCl) --> light green Mo (+H2O) --> orange Mo
Light green Mo + KMnO4 --> MoO4(2-) (probably) + MnO2 (+Na2S2O5) --> MoO4(2-) + Mn2+
Orange Mo + KMnO4 --> MoO4(2-)
Orange Mo + Na2MoO4 --> molybdenum blue
Orange Mo + more H2O (or NaOH) --> colourless Mo
Na2MoO4 + more Zn than in first reaction + conc. HCl --> orange Mo (+ HCl) --> very pale red Mo (+Na2MoO4) --> light green Mo
Very pale red Mo + KMnO4 --> MoO4(2-) + MnO2 (+Na2S2O5) --> MoO4(2-) + Mn2+
Na2MoO4 + Zn + H2SO4 --> dark green Mo --> orange Mo (but slightly different shade than first orange Mo) (+H2SO4) --> orange-green Mo
Seems like this molybdenum species forms complexes with sulphates and chlorides and have reducing properties
Edit: I found, what is the light green compound is: https://www.imoa.info/molybdenum-uses/molybdenum-chemistry-uses/molybdenum-oxidation-states.php
[Edited on 2-6-2019 by Bedlasky]
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Discussion
So I've done some research.
I completed reaction mechanism which is shown on picture. I gave them working names (except first two ones):
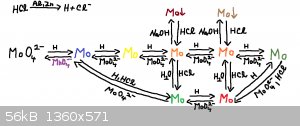
Molybdate-->Molybdenum blue-->Yellow molybdenum-->Orange 1 molybdenum (below it is green molybdenum)-->Orange 2 molybdenum (below it is
red molybdenum)-->Dark green molybdenum
All these molybdenum compounds are oxidized by molybdate in to higher molybdenum species (except molybdenum blue and molybdate).
All these molybdenum compounds are reduced by nascent hydrogen in to lower molybdenum compounds (except dark green molybdenum).
Orange 1 and Orange 2 form in concentrated HCl chloride complexes.
Orange 1 and Orange 2 form in basic solution some hydroxide, oxide-hydroxides or hydrated oxides soluble in HCl (maybe dark green and yellow too
create hydroxides, I didn't test it).
Solution of green molybdenum is stable, but red molybdenum is slowly oxidize (few days). Dark green is oxidize far more quickly (after few minutes in
hot water bath is apparent colour changing).
Green and red molybdenum are oxidized after evaporation of HCl in to molybdenum blue.
Reduction of molybdate is very sensitive to amount of used zinc. It's very easy to add too much zinc powder and you get different compound. To
preparation of higher molybdenum species is better to use aluminum foil.
Yellow molybdenum I prepared only once acidently during analysis.
Green molybdenum react with dark green molybdenum to form red molybdenum.
Analysis
In tuesday I've done some analysis. Sadly I didn't have much time, so I analysed only yellow, orange 1 and dark green molybdenum. I chose
manganometry, but it was hard determine the equivalence point because of oxidation of chlorides. I weighed sodium molybdate on analytical scales and
zinc powder to two decimal places. I used little amount of MnCl2 to inhibition chloride oxidation.
Here are results:
Yellow molybdenum is mixed-valence species with average oxidation number 5,8. Maybe molybdenum bronze or something like that?
Orange 1 molybdenum have average oxidation number 5,14 - so probably it is Mo(V) species.
Dark green molybdenum have average oxidation number 3,82.
Orange 2 molybdenum is somewhere between 5,14 and 3,82.
Little discusion:
I read some materials:
Molybdenum in lower oxidation states
Molybdenum chloride complexes
Molybdenum in different oxidation states (VI-III)
Reduction of Mo(VI) and description of molybdenum in different oxidation states
Orange 1 molybdenum is probably [Mo2O4]2+ or maybe some chloride complexes ([MoOCl]2+; [MoOCl2]+; or [MoOCl3]).
Green molybdenum is probably [MoOCl5]2-.
This agrees with literature and with my analysis.
Dark green molybdenum resembles its colour and some properties with [Mo(H2O)Cl5]2- and [MoCl6]3- (according to literature). But this is not
corresponding with my analysis. But there is some explanation - 1. Mo(III) is quickly oxidized by air 2. Maybe was reduction incomplete (mixed
solution of Mo(III) + Mo(IV)).
According to some sources [MoCl6]3- which exists in HCl > 4,5M is red - but I never prepare red solution from dark green molybdenum adding
concentrated HCl.
If are these presumptions right, orange 2 molybdenum is [MoO(OH)]+ and red molybdenum is [MoOCl]+.
What is yellow molybdenum I don't know. Maybe molybdenum bronze - these compounds have a wide range of colours.
 
[Edited on 21-6-2019 by Bedlasky]
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I posted article about this reaction on my website in czech and english.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Interesting post and work Bedlasky.
I tried to synthesis some of your colourful molybdenum compounds. I started with molybdenum blue and oxidised to molybdenum acid with H2O2.
As my HCl was not available I used dilute sulphuric acid, sodium chloride. and zinc. It first seemed to produce some wisps of molybdenum blue
round the zinc but later it turned a muddy dark greenish colour. I added more zinc but it remained cloudy and dark greenish in colour. I will try
again with the correct reagents. I have some more small pieces molybdenum (mounting wire of the filament of a MO magnetron) slowly dissolving in
12%H2O2 to make ammonium molybdate.
So now I have a waste solution containing <100mg of molybdenum. I think its not significantly poisonous but I am reluctant to flush it down
the sink. Perhaps I could precipitate the molybdenum as the sulphide with hypo.
PS: Sodium thiomolybdate Na2MoS4 is an interesting colourful dark red. Its prepared by the action of hydrogen sulphide upon a solution of sodium
molybdate .
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Reduction by zinc dust is very sensitive and if you add too much zinc you reduce all molybdenum to III oxidation state (which is very dark green). So
this is probably what happened to you.
For making Mo(V) and Mo(VI/V) compounds is better to use aluminium foil because it have relatively small surface comparing to zinc dust, so aluminium
foil generate less nascent hydrogen. If you want to use zinc dust, you must use only very very tiny amount of it in more concentrated molybdate
solution.
For making Mo(IV) and Mo(III) is better zinc dust. If you want to use aluminium foil, you must add very large amount of it - so reduction with
aluminium foil is really annoying (especially on III oxidation state). If you want to make Mo(IV) solution you must be carefull with addition of zinc.
Add it only in small portions. You can also test if the reduction is complete - just add few drops of your solution in concentrated hydrochloric acid.
If solution is red, then reduction on Mo(IV) is complete, if solution is greenish brown, then you must add more zinc. I use similar test on Mo(V).
There are also other reducing agents. For example:
Molybdenum blue can be formed by reaction of sodium molybdate with Fe2+, Na2S2O3, ascorbic acid, glucose, fructose, sucrose or Na2S2O4 in acidic
solution.
Mo(V) can be formed by reaction of sodium molybdate with excess of SnCl2 or TiCl3 in acidic solution. I suppose that V2+ and Cr2+ salts also works.
Quote: Originally posted by wg48temp9  |
So now I have a waste solution containing <100mg of molybdenum. I think its not significantly poisonous but I am reluctant to flush it down
the sink. Perhaps I could precipitate the molybdenum as the sulphide with hypo. |
If you have solution which contains only few hundred milligrams of molybdate, than you can flush it down the sink. Molybdenum isn't that toxic for
environment as another heavy metals like Pb, Tl, Hg, Co, Ni, Cu etc. Molybdates can be precipitated by concentrated solution of CaCl2.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Very interesting, especially your scheme of all oxidation states.
I myself have done experiments with Mo as well, and I also observed the yellows, reds and blues. I even did an electrolysis experiment of molubdate,
dissolved in dilute HCl. At the cathode, a red compound is formed (I have this experiment on my website):
https://woelen.homescience.net/science/chem/exps/electrolysi... (first experiment)
I also did experiments with ascorbic acid and hypophosphite as reductor:
https://woelen.homescience.net/science/chem/exps/colorfulmol...
Finally, I dissolved molybdenum metal in HNO3. This experiment has unexpected results:
https://woelen.homescience.net/science/chem/riddles/Mo+HNO3/...
Molybdenum has a remarkable, and also still poorly understood chemistry.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Your solution from electrolysis looks exactly like Mo(V) or Mo(IV) solution. I read that molybdates can be electrolyticaly reduced even at III
oxidation state.
The precipitate from dissolving molybdenum in HNO3 maybe dissolves in concentrated HCl. Lower oxidation states of molybdenum formed stable chloride
complexes which are definitely soluble in acidic solutions. HNO3 can oxidize this lower oxidation states of molybdenum in to molybdate (I once tried
add nitrate in to Mo(V) solution), but you probably used excess of molybdenum.
I will definitely post few articles about molybdenum chemistry in future. I have plenty of photos from various experiments with sodium molybdate. I
also want to try titanometric determination of molybdate and preparation of (NH4)2[MoOCl5].
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by Bedlasky  | | The precipitate from dissolving molybdenum in HNO3 maybe dissolves in concentrated HCl. Lower oxidation states of molybdenum formed stable chloride
complexes which are definitely soluble in acidic solutions. HNO3 can oxidize this lower oxidation states of molybdenum in to molybdate (I once tried
add nitrate in to Mo(V) solution), but you probably used excess of molybdenum. |
I'll make some HNO3 again, and try this experiment again and then add some concentrated HCl after making the precipitate. In another experiment, I'll
mix the HNO3 and HCl beforehand (making aqua regia) and add molybdenum metal to that mix.
I did not use excess Mo in that experiment, but excess HNO3 (actually, the excess amount was quite large, the liquid in the pictures is concentrated
HNO3, while there is just a spatula of Mo-powder).
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  | Reduction by zinc dust is very sensitive and if you add too much zinc you reduce all molybdenum to III oxidation state (which is very dark green). So
this is probably what happened to you.
For making Mo(V) and Mo(VI/V) compounds is better to use aluminium foil because it have relatively small surface comparing to zinc dust, so aluminium
foil generate less nascent hydrogen. If you want to use zinc dust, you must use only very very tiny amount of it in more concentrated molybdate
solution.
|
Actually initially I used thick aluminium strip cut from a clean tart base but it was too slow so I switched to a strip of zinc foil. The zinc was
more reactive. Unfortunately I was forced to leave the experiment unattended.
What I thought would happen is the molybdenum would be slowly reduced and therefore go though the colours you displayed. That may have happened
while it was unattended.
I wanted to repeat the video shown in http://www.sciencemadness.org/talk/viewthread.php?tid=82505#...
but with all the colour changes.
PS: I see that my video has been down loaded and presumably viewed 368 times. It took some effort to capture that effect so to see that it has
been viewed 368 times makes my effort worthwhile. 
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by woelen  | | I did not use excess Mo in that experiment, but excess HNO3 (actually, the excess amount was quite large, the liquid in the pictures is concentrated
HNO3, while there is just a spatula of Mo-powder). |
This is mystery for me. If you use excess HNO3 it should oxidize Mo in to VI oxidation state (in theory). I once tried if I precipitate strontium
molybdate(IV) with strontium nitrate and solution turned in to colourless. I today tried few tests.
1. I mixed sodium molybdate, hydrochloric acid and aluminium foil to form reduced molybdenum compounds. Then I added some potassium nitrate and
solution turned in to the yellow with formation a little bit of NO2 gas. It didn't visibly react with KMnO4 (only some MnO2 was formed due to reaction
with HCl). Yellow colour is probably caused by NOCl/Cl2/NO2. Solution smelled of chlorine.
2. I mixed sodium molybdate, sulfuric acid and zinc dust to form reduced molybdenum compounds. Then I added some potassium nitrate and solution turned
in to the orange with formation a little bit of NO2 gas. Maybe is orange colour cause by NO2? But colour is too much intense in my opinion. Solution
smelled of chlorine, which is quite surprising for me. How smell NO2? Have NO2 similar smell?
I've got one other idea for test your precipitate from HNO3. Try it dissolve in hot acidified phosphate solution for the case that it is compound of
molybdenum in VI oxidation state. Phosphates form very stable yellow complex with molybdates. Phosphate-molybdate complex is better test then
peroxide-molybdate complex because phosphate haven't any oxidation properties.
Here is also some literature:
https://books.google.cz/books?id=aFSnXpONJEoC&pg=PA74&lpg=PA74&dq=%5BMoOCl5%5D2-&source=bl&ots=Vme0BHAW8v&sig=ACfU3U3UQYi94Ss8
57CiqpsGRMxm0NCnsQ&hl=cs&sa=X&ved=2ahUKEwiNms783sviAhVGwMQBHXzRCKAQ6AEwCHoECAcQAQ#v=onepage&q=%5BMoOCl5%5D2-&f=false
Quote: Originally posted by wg48temp9  |
What I thought would happen is the molybdenum would be slowly reduced and therefore go though the colours you displayed. That may have happened
while it was unattended. |
Aluminium foil must be dissolved at least in 1+1 HCl. If concentration of HCl is lower, then dissolving is very slow. You want rapid dissolving. Be
careful because reaction between Al and HCl is quite exothermic.
[Edited on 17-2-2020 by Bedlasky]
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Molybdates of zinc and iron are insoluble and aluminium molybdate is very sightly soluble. So zinc or ferrous salts might be used to produce
insoluble waste from soluble molybdate waste. Probable zinc is best to avoid ferrous ferric problems and it will be necessary to make certain
the molybdenum is in the Vl oxidation state.
It also probably means that zinc, iron and aluminium tends to passivate in molybdate solutions.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I tried the experiment with HNO3 again.
I took 2 ml of room temperature HNO3 (concentration not known exactly, pale yellow home-made stuff, close to azeotropic concentration, but might be a
little less or more).
To this HNO3 I added a small amount of powdered Mo-metal (reagent grade, 99.9%) and swirled. Initially nothing happens, but after half a minute or so,
a reaction sets in, which becomes more and more violent. A lot of NO2 is produced and a brown solid is dispersed in the acid. Quickly the reaction
subsides again and a dark brown turbid liquid is obtained. The dark brown material is a solid, finely dispersed in the acid.
I heated the liquid until it started boiling. This causes production of more NO2 and the color of the solid slowly changes from dark brown to lighter
brown/red until it becomes beige/brown. It does not become completely white.
In another test tube, I took 1 ml of this home-made HNO3 and added 2 ml of conc. HCl (37%). To this mix, I added a small amount of Mo-metal.
Initially, nothing happens. The metal is finely dispersed in the liquid and a dark grey liquid is obtained. After a minute or so, the color shifts
from grey to reddish brown and a reaction sets in. The material then dissolves, in a few tens of seconds and a lot of orange/yellow gas is produced
(must be ONCl, I recognized its smell). The liquid turns deep red and becomes clear. No solid matter is dispersed in the liquid.
I boiled this liquid as well after the reaction. While boiling, the liquid becomes lighter and lighter. Finally it becomes yellow, the typical color
of aqua regia which has aged. On dilution the liquid becomes colorless (aqua regia has a yellow color, due to dissolved ONCl, which hydrolyses to
colorless compounds on dilution with water). So, with aqua regia you get a red compound, which is oxidized to a colorless compound of molybdenum on
further heating.
Finally, I added some conc. HCl to the test tube with HNO3 and the beige solid in it. The HCl causes this solid to dissolve. It does not dissolve
immediately, but on gentle heating it dissolves fairly easily and the solution is yellow (color of aqua regia, but with too much HNO3 in it).
So, on further heating the molybdenum indeed is oxidized to its +6 oxidation state, giving white/colorless compounds, but in the initial reaction,
even when the temperature is quite high, there is a brown/red compound. In HNO3 it is an insoluble compound, in HNO3/HCl it dissolves.
I find it quite remarkable, that even in large excess concentrated HNO3 the molybdenum is not immediately oxidized to the +6 oxidation state. In the
concentrated acid, the red/brown compound can exist without being oxidized further. Only on strong heating (boiling azeotropic acid) it is further
oxidized.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Very interesting results Woelen!
It's interesting that oxidation is gradual. I am interested what this brown stuff is. Maybe it's some molybdenum oxide? You could find out easily
oxidation state of Mo in it. Just filtred off this brown precipitate, wash it with little bit of water and dissolve it in concentrated HCl. Mo(V)
forms green complex, Mo(IV) red complex, Mo(III) brown complex. I personally guess that brown precipitate is probably hydrous Mo2O5 or another Mo(V)
compound, because Mo(IV) and Mo(III) have strong reducing properties (they are oxidized even by oxygen), but maybe I am wrong. This test can be also
used for analysis beige/brown precipitate.
It's interesting how complex formation (and also formation of stronger oxidant as NOCl/Cl2 mixture) can affect reaction.
May I ask where did you get molybdenum powder?
Thanks for sharing your results!
[Edited on 18-2-2020 by Bedlasky]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Maybe a complex with NOCl is formed? As I checked for presence of a lone pair on NOCl, I found that Wikipedia says:
| Quote: |
Nitrosyl chloride is used to prepare metal nitrosyl complexes. With molybdenum hexacarbonyl, NOCl gives the dinitrosyldichloride complex:[6]
Mo(CO)6 + 2 NOCl → MoCl2(NO)2 + 6 CO
[6] Johnson, B. F. G.; Al-Obadi, K. H. (1970). "Dihalogenodinitrosylmolybdenum and Dihalogenodinitrosyltungsten". Inorg. Synth. 12: 264–266.
doi:10.1002/9780470132432.ch47.
|
Who knows what it is capable of forming in an aqueous environment?
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I tried reaction between acidified ammonium heptamolybdate and sodium nitrite and there wasn't visible reaction. So yellow colour is caused by NOCl.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  | Very interesting results -------
May I ask where did you get molybdenum powder?
Thanks for sharing your results!
[Edited on 18-2-2020 by Bedlasky] |
Its available on ebay uk.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I obtained my Mo-powder several years ago, indeed from eBay, but I do not know the seller anymore. It was either German or British.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
http://svetprvku.cz/12-prvky
Molybden - prášek
Čistota 99,9%
6 CZK (0,25 EUR) per 1 g
49 g in stock
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I did few experiments with Mo powder today. And I have few interesting observations.
Reactions with acids and peroxides:
31% HCl - Mo very very slowly dissolved in hot water bath to form green [MoOCl5]2-
38% H2SO4 - Without reaction
65% HNO3 - Mo very quickly dissolved to form white powder and yellow solution. After dilution solution became colourless. White powder is probably
H2MoO4.
35% H2O2 - Mo very quickly dissolved even at room temperature to form yellow [MoO3(O2)]2- complex.
Reaction with acids and ammonia mixtures:
HCl + H2O2 - Mo very quickly dissolved to form yellow peroxide complex
HCl + HNO3 - Mo quickly dissolved in hot water bath to form yellow solution. Upon dilution yellow colour disappeared - so solution contained Mo(VI).
HCl + (NH4)6Mo7O24 - Mo quickly dissolved in hot water bath to form green [MoOCl5]2- complex
H2SO4 + H2O2 - Mo very quickly dissolved to form yellow peroxide complex
H2SO4 + (NH4)6Mo7O24 - Mo dissolved in hot water bath to form brown solution of [Mo2O4]2+. It seemed to me that reaction with HCl/heptamolybdate
mixture was faster.
NH3 + H2O2 - Mo dissolved firstly to form reddish brown solution of [Mo(O2)4]2-. After heating solution became colourless due to decomposition of
peroxide complex in to molybdate.
[Edited on 22-3-2020 by Bedlasky]
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Preparation of Phosphomolybdic Acid
Below is a prep of Phosphomolybdic Acid. Its more soluble than Molybdic Acid so it may be useful starting compound for other Mo compounds

I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
wg48temp9: Thanks for nice paper  . .
I experimented today little bit with dissolving Mo metal again. Yesterday I swapped test tube with HNO3 and aqua regia, so observations was different
today (I'll edit yesterday post and correct it).
Heating Mo with 65% HNO3 - white powder of H2MoO4
Reaction of Mo with aqua regia - brown solution, after dilution it's nearly colourless.
Mo reacts very different with dilute nitric acid (I used 1+1 HNO3). It reacts vigorously even without heating to form brown powder. This brown powder
is soluble in HCl to form orange solution. What is this compound I really don't know. I thought that maybe hydrated MoO2 or some Mo(VI)/Mo(V) oxide,
but I tried to oxidize Mo(IV) and molybdenum blue with HNO3 and both compounds was oxidized in to Mo(VI). So I am really confused what is it.
Mo is very quickly dissolved by mixture of 65% HNO3 and NaH2PO4 to form yellow solution.
Mo doesn't react with conc. sulfuric acid.
Mo very vigorously reacts with HNO3/KBr mixture
Mo is very slowly attackted also by dilute HCl (I used 1+1 HCl) to form green solution.
Mo is very quickly dissolved by mixutre of H2O2 with 4% NaOH, firstly to form red solution, which become after heating colourless.
Mo doesn't react with bleach
Addition of molybdate in to the dilute HNO3 doesn't affect reaction with Mo metal.
Quick summary:
Mo metal can be dissolved in:
1. conc. HNO3, dilute HNO3, conc. HCl, dilute HCl
2. aqua regia, HBr/HNO3 mix., H3PO4/HNO3 mix., HCl/molybdate mix.
3. almost any acidic or basic mixture of H2O2, H2O2 itself
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Below is a brief paper on the some salts of Phosphomolybdic Acid. Several salts of monovalent elements including potassium and ammonia are sparingly
soluble while the salts of sodium and di or trivalent elements are all soluble.

I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
wg48temp9: Thank you very much for your post! You help me to explain one mystery. A few days ago I want to take a picture of sodium phosphomolybdate
solution. I dissolved some Na2MoO4 in water, add tiny amount of K2HPO4 and add few drops of conc. HCl. I obtained yellow solution. But when I added
more phosphate, I obtained yellow precipitate, which was surprise for me. Now I know why. I used POTASSIUM hydrogen phosphate. When I repeated this
experiment today, I used NaH2PO4 instead of K2HPO4. I added just a tiny amount of phosphate and obtained yellow solution. When I added more phosphate,
solution became colourless - which corresponds with my previous observations.
So sodium phosphomolybdate is interesting reagent for K+, NH4+ and Rb+ test.
Btw - when you add K+, NH4+ or Rb+ salt in to the solution of sodium silicomolybdate, you obtain also yellow precipitate. Reaction with Rb+ is
immediate, while reaction with NH4+ and K+ requieres heating.
|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I have few new observations about dissolving Mo metal in acids:
85% H3PO4 - no reaction
99% HNO3 - In cold acid there is no reaction, in hot acid there is formation of small amount of nitrogen oxides. If you add small amount of water in
to this mixture, vigorous reaction take place and lots of MoO3 precipitate is formed.
1+1 HNO3/NaH2PO4 mixture - Formation of brown solution.
|
|
|
| Pages:
1
2 |