postart
Hazard to Self
 
Posts: 59
Registered: 29-6-2010
Member Is Offline
Mood: No Mood
|
|
Calcium Acetate PAA (dry distillation)
Hay all, I have obtained a few liters of phenylacetic acid and have decided to attempt a dry distillation to yeild the ketone. I am using the
procedure below from the rhodium archive as a reference. I do not like the idea of handling large ammounts of lead and don't want any moonrocks left
in the flask lol. Anyways this is why I have decided to use calcium acetate in place of lead acetate. Charing is the biggest concern with this dry
distillation. I will use a light vacc from a water aspirator to try and prevent destruction. Has anyone ever run this distillation with success? Any
last thaughts, comments, or advice before my first run would be greatly appreciated?
From Rhodium:
Lead Acetate Method [6]
Place 1000g phenylacetic acid and 3000g anhydrous (or trihydrate) lead acetate in a distillation apparatus and heat. First an amount of water will
distill, and next phenyl-2-propanone in this destructive distillation, which requires liberal application of heat. The distillate will separate into
two layers. The organic layer is separated and redistilled to give pure phenyl-2-propanone, bp 105°C/10 mmHg or 216°C at atmospherical pressure.
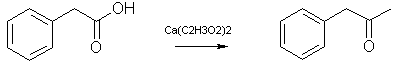
|
|
|
spirocycle
Hazard to Others
  
Posts: 197
Registered: 29-9-2010
Member Is Offline
Mood: No Mood
|
|
how is it that you can obtain a few liters of PAA but don't know how to process it?
|
|
|
postart
Hazard to Self
 
Posts: 59
Registered: 29-6-2010
Member Is Offline
Mood: No Mood
|
|
What do you mean? I pretty much outlined how I'm going to process it, just thaught I would ask if anyone has done the calcium variation of which
lititure is not plentiful.
[Edited on 7-11-2010 by postart]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Use the Search engine, Luke.
The subject has been discussed a number of times before. Find one of those threads, ask the question there, and you might get an answer other than
being sent to detritus.
|
|
|
postart
Hazard to Self
 
Posts: 59
Registered: 29-6-2010
Member Is Offline
Mood: No Mood
|
|
Sorry, I looked up some of them but wanted to discuss the Calcium variation in a seprate thread if thats alright. I mean if somone was searching for
this specific variation it could be a very useful thread?
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Liters you say??
According to the Merck Index, the melting point is 76.5 C. Solids are generally not measured in liters. If a liquid, well...
|
|
|
postart
Hazard to Self
 
Posts: 59
Registered: 29-6-2010
Member Is Offline
Mood: No Mood
|
|
whopse kg you know what I meant lol.
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
I did a couple dry distillation experiments with calcium phenyl acetate and calcium acetate in the early 80's. PAA and P2P were both not listed at
that time. I found CaO worked to make the salts, but CaCO3 didn't work because it reacted too slow especially with the PAA. I mixed in some fused
sodium acetate with the calcium salts since one process I found in a chemistry book said it facilitated the production of acetone from calcium acetate
alone. From my experiments, dry distillation of the calcium salts made using CaO worked. It produced a sweet smelling liquid which smelled just like
pure P2P , but trying the dry distillation when trying to make the calcium salts using CaCO3 didn't work too well. It produced a small amount of
burnt smelling distillate, instead of a larger amount of sweet smelling distillate in the CaO experiments.
[Edited on 7-11-2010 by Vogelzang]
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
You can find a lot of information using Google books. CaCO3 is used in the first link. It looks like you might have to boil the reactants long
enough to get them to react. CaCO3 is almost insoluble in water. It looks like calcium phenylacetate might be soluble enough in water to separate
it from excess CaCO3 by filtration.
CaO reacts faster with the acids compared to CaCO3.
P2P easily forms a sodium bisulfite addition product.
http://books.google.com/books?id=j38FAQAAIAAJ&pg=PA7&...
http://books.google.com/books?id=ocg3AAAAMAAJ&pg=PA1185&...
[Edited on 8-11-2010 by Vogelzang]
|
|
|
postart
Hazard to Self
 
Posts: 59
Registered: 29-6-2010
Member Is Offline
Mood: No Mood
|
|
Thanks for the help I was going to just plug in calcium acetate to the lead variation. If possible I was thinking 4kgs calcium acetate and 1kg PAA
heated untill P2P distills over @ aspirator pressure. should be theoredically possible.
[Edited on 8-11-2010 by postart]
[Edited on 8-11-2010 by postart]
[Edited on 8-11-2010 by postart]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Yeah, you should definitely use a full kilo of PAA, right away. No trial runs; just whammo!
|
|
|
postart
Hazard to Self
 
Posts: 59
Registered: 29-6-2010
Member Is Offline
Mood: No Mood
|
|
just keeping it simple.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Indeed. Running a 7 mole synthesis that you don't understand without a small scale
trial is about as simple as anything I can think of.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
postart, a chemist would not start with such a scale without some trial runs. Are you a chemist or are you a cook? Cooks aren't welcome here.
I weep at the sight of flaming acetic anhydride.
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
This procedure has been on the internet for about a decade:
http://www.erowid.org/archive/rhodium/chemistry/p2p.paa-ca.h...
you really should pay close attention to what's been advised in this thread  You are risking a lot needlessly by not using proper scientific procedures
You are risking a lot needlessly by not using proper scientific procedures  Small
scale trials minimize the dangers implicit to what you're contemplating Small
scale trials minimize the dangers implicit to what you're contemplating  One
mistake can be disastrous One
mistake can be disastrous  Good advice like you've gotten is priceless Good advice like you've gotten is priceless 
Chemistry is our Covalent Bond
|
|
|
Vogelzang
Banned
Posts: 662
Registered: 26-4-2008
Member Is Offline
Mood: No Mood
|
|
At least if he burned up all of his PAA, he probably wouldn't get caught and go to jail. Also, anyone who says that they have liters of PAA probably
doesn't really have any at all.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
| Quote: | | Yeah, you should definitely use a full kilo of PAA, right away. No trial runs; just whammo! |
*bursts into laughter for an extended period of time*
He has no phenylacetic acid. What probably happened is he assumed it was a liquid because acetic acid is a liquid. A simple mistake a non
chemist would be likely to make.
Hey, why don't you take a picture of some of this supposed PAA? Perhaps then we would be more apt to believe you.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
i have not run these reactions before however i have run other dry distillations and found that a carrier gas bled in quite slowly is almost essential
when working at the 450C mark, otherwise your products have too much residence time in the hot environment. Bubble the exit gas after condensing
through some ice water for a flow meter. Nichrome coiled around a borosilicate glass tube within which your mixture lies is the best way to heat these
temperamental procedures. If you are unfamilar with a new procedure you can THROW CAUTION TO THE WIND and leave the tube unlagged for a few runs so
observations can be made. Hopefully it needn't be mentioned that the tube should incline slightly down in the direction of the gas flow. Use a large
bore and long hose between your gas bottle and your system to ensure the gas haas equilibrated to room temperature. Calcium salts smell nicely like a
bakery.
I would start at around 10g and a very small setup probably ~20mm diameter tube. Remember to let the system fully cool before washing it out otherwise
your tube could readily crack.
Aromatic Ketones from calcium salts is a very old method from around 100 years or so ago, however it remains useful in some circumstances, examples of
which completely escape me, ah that's right, the hardier di-aromatic ketones.
|
|
|
TIETSE
Hazard to Self
 
Posts: 53
Registered: 21-8-2008
Member Is Offline
Mood: No Mood
|
|
another cook lost and lazy to utfse 
Attachment: Carboxylate Salts to Ketones.pdf (790kB)
This file has been downloaded 1724 times
Attachment: Decarboxylation Studies. II. Preparation of Alkyl Phenyl Ketones1,2.pdf (431kB)
This file has been downloaded 2203 times
Attachment: Mixed.Ketones.From.Mixed.Calcium.Salts.pdf (155kB)
This file has been downloaded 1878 times
Attachment: Mechanism of ketonic decarboxylation. Pyrolysis of calcium decanoate.pdf (753kB)
This file has been downloaded 4831 times
"Minds are like parachutes, they only function when they are open. "
|
|
|