Keras
National Hazard
   
Posts: 938
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Acetylene Trimerization to Benzene (again)
I found a thread about this subject, but it is old (2002). So I decided to resurrect it.
Has anyone attempted this (i.e. 3 C2H2 → C6H6)?
The original thread referenced March's Advanced Organic Chemistry. The 7th version states (cf. attached image) that a Ni(CN)2 or
any Ni(II) compound should work as a catalyst (cf. Reppe), but the book doesn’t state anything about temperature.
However, I also found a 1968 patent about low temp benzene synthesis from acetylene where a mixture of chromium trioxide and aluminia is mentioned as
a working catalyst, too (pdf in attachement).
Anyone interested by testing this setup? Acetylene is readily made from calcium carbide, chromium trioxide shouldn't be too hard to get from more
commonly available Cr(III) compounds. Working with low temp acetylene seems a good idea, given the propensity of the gas to explode.
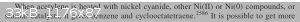
Attachment: Low Temperature Conversion of Acetylene to Benzene.pdf (528kB)
This file has been downloaded 463 times
[Edited on 17-4-2019 by Keras]
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
There is an interesting video on youtube about it, I am sorry if it has been posted before, looks quite a bit dirty
https://www.youtube.com/watch?v=_HNUjoNEmtc
The paper is interesting although I can´t add anything. Go try it! I´d love to see a good benzene synth from acetylene! Still hoping for a good
continious production of benzene in the lab!
|
|
|
Keras
National Hazard
   
Posts: 938
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Uh, the computer voice is horrible :p Also, that’s precisely what I'd like to avoid, i.e. high temp. Besides, did you watch the final notes?
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
Quote: Originally posted by Keras  |
Uh, the computer voice is horrible :p Also, that’s precisely what I'd like to avoid, i.e. high temp. Besides, did you watch the final notes?
|
Yeah I did(again), wished I had a cleaning slave. I only got my distilling darlek. I also had a quick look in the paper you posted and really liked
the low temp <100°C used! I really think your project is worth it´s time, may I can help you with some Chromium salts?
|
|
|
Heavy Walter
Hazard to Others
  
Posts: 127
Registered: 17-12-2015
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
benzene synthesis
Hi
I did the synthesis years ago. Completely quantitative.
See the setup involved.
I used vanadium pentoxide, cold traps (ice/salt; acetone/dry ice and liquid N2).
My purpose was to obtain deuterated benzene, so I used heavy water.
Any doubts?

|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
Really nice Heavy Walter, really nice! I´m jealous.
So was the benzene totally deuterated! and you were left with deuterated Ca(OdH)2? what did you do with these components? I am even more interested
now!
|
|
|
Keras
National Hazard
   
Posts: 938
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Heavy Walter  | Hi
I did the synthesis years ago. Completely quantitative.
See the setup involved.
I used vanadium pentoxide, cold traps (ice/salt; acetone/dry ice and liquid N2).
My purpose was to obtain deuterated benzene, so I used heavy water.
Any doubts? |
Why would you use cold traps?
Also, what was your yield? Any idea?
|
|
|
Keras
National Hazard
   
Posts: 938
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
It’s very nice of you, but I think I can cope. I do have a few hundred grams of chromium III chloride somewhere, which I can easily turn into
chromium VI oxide using hydrogen peroxide, lye and hydrochloric acid (e.g.: here). I appreciate your offer, though! 
Now, I don’t have aluminia, and I wonder if CrO3 must be mixed with it, or deposited on its surface, to be efficient.
[Edited on 18-4-2019 by Keras]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Great video by astral chemistry but no workup of the product and final yeild.would like to know the litres/hr from running that setup.if the
alumina/cr03 method makes a cleaner product that might make it worth doing.
|
|
|
Heavy Walter
Hazard to Others
  
Posts: 127
Registered: 17-12-2015
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
Hi Dr Scabs,
My purpose was to explore the trimerization in order to produce commercially deuterated benzene.
Was nice try but commercially bad, due to very low price of the product in the market.
Hi Keras
I used industrial carbide and the traps were useful to stop heavy water vapors, air traces and some other stuff.
My notebook is not at hand now but I produced 5 mL, extremely pure C6D6.
During the operation there were two accidents: a stopcock flew away due a sudden overpressure and at the end, when pouring the excess of liquid N2 in
a large dewar, the small dewar exploded when tilting it.
I employed those commercial small dewars for keeping warm food for kids and tilting it generated stresses on it.
|
|
|
Keras
National Hazard
   
Posts: 938
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Thanks. I’ll attempt that synthesis next week. I plan to do it using acetylene dissolved in acetone, since I don’t like the idea of working with
the gas.
Chromic acid doesn’t seem to react with acetone, so I should be fine.
[Edited on 25-4-2019 by Keras]
|
|
|
DrScrabs
Hazard to Others
  
Posts: 123
Registered: 13-3-2018
Location: Laputa
Member Is Offline
Mood: Still evaporating..
|
|
Please remove the previous spam/advisment post, I and maybe some other members are interested in this procedure, keep it up!
@Heavy Walter If your yield has been nearly quantitative I´d love to have a procedure for it! Please contribute it to us!
|
|
|