Gearhead_Shem_Tov
Hazard to Others
  
Posts: 167
Registered: 22-8-2008
Location: Adelaide, South Australia
Member Is Offline
Mood: No Mood
|
|
Grouping of compatible chemicals for storage
I've been scratching my head over how to interpret this table from Prudent Practices in the Laboratory:
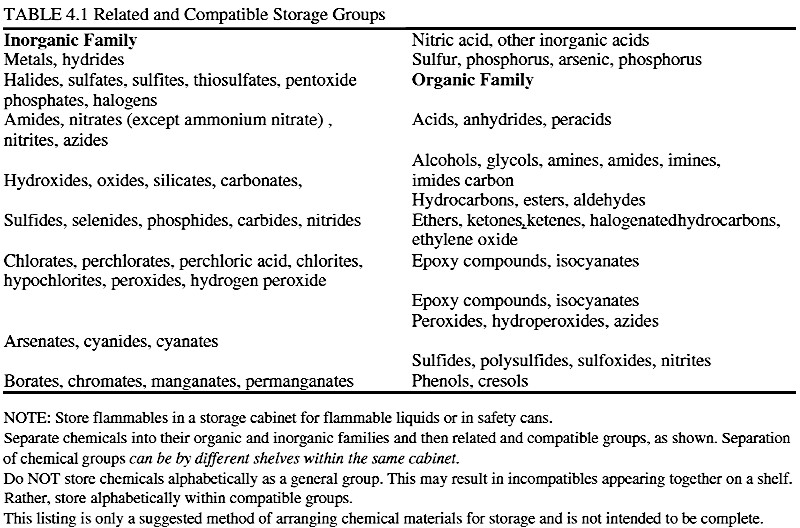
The organics confuse me particularly (why are epoxies & isocyanates listed twice?), but I strongly suspect there are subtleties here that I'm
missing. For instance, exactly how many groups are shown? By one count -- the number of capitalised lines -- it'd be nineteen, but just eleven by
whitespace separation. Which is right?
And since I'm still a rank beginner, what is the reasoning behind each group? And once they are grouped on shelves, which groups would you
not put below or above one another in case of leaks or wafting vapours?
-Bobby
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
I've got a feeling that the other compounds in those groups containing epoxies and isocyanates may not be compatible with each other.
In terms of spills, if you can get away with it: don't put anything above other things  (I know that's no easy task) (I know that's no easy task)
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
That list looks very incomprehensive and poorly done, one could add things like not to store ammonium salts with chlorine oxidants (TCCA, NaDDC),
chlorites, hypochlorites, chlorates, or permanganates. From the looks of it, the left side materials are alright to store with each other as long as
they are in their group. And don't mix the stuff on the left with the stuff on the right.
Usually, chemicals from those type of lists are said to be incompatible because they ignite, give off poison, form other dangerous compounds, or even
explode.
Some examples from that list would be sulfides or selenides and strong acids form H2S and H2Se two extremely poisonous compounds especially the
latter. Phosphides and acid makes phosphine which in high enough concentrations ignites on contact with air and is extremely poisonous. Glycols and
permanganates ignite after some time, sulfoxides like DMSO and permanganates (KMnO4) ignite on contact, etc.
But that list is poorly written and I wouldn't try to connect the groups. I can't think of anything dangerous ethers and sulfides are supposed to do,
for example.
So, I would rather look at individual MSDS sheets stating incompatability of the chemicals you already have, or chemical hazard dictionaries (there
are plenty of those).
|
|
|
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Hmm DMSO ignites on contact with KMnO4 must try that sometime
Anyway just split your chemicals like this
Oxidants (NaNO3, KClO3, KMnO4) (Give the ammonium ones their own box so there separated)
Flammables (Hexane, Al Powder, Ethanol etc.)
Strong Acids (H2SO4, HNO3, HCl)
Bases (KOH, NaOH, etc.)
General (CuSO4, Fe2O3, CH3COONa)
|
|
|
Anders2
Banned
Posts: 39
Registered: 4-9-2010
Member Is Offline
Mood: No Mood
|
|
I had my own idea for categories:
1. Oxidizing Acids
2. Reducing Acids (such as HBr or acetic)
(also store with hydrocarbons, including organic amines)
3. Oxidizing bases (such as Ca(OCl)2)
4. Reducing Bases (would include NaOH, Mg3N2, KCN)
(also would include Na, Mg)
5. Explosives with vulnerable oxidizing component (organic peroxides)
6. Explosives with vulnerable reducing component (hydrazinium nitrate)
7. Metals/ transition salts
These categories would allow efficient grouping to ensure no incompatable chemicals were stored with eachother. Can you think of two incompatable
chemicals that could fit into the same category?
[Edited on 17-9-2010 by Anders2]
|
|
|