un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Anyone got ideas? MCPA to 2,5-dihydroxytoluene...
I was looking around for the agricultural products I thought I recalled seeing as the dimethylamine salts (in conjunction with something else) and
found that I recalled correctly, the fertilizer/herbicides (weed & feed type) such as MCPA {aka (4-Chloro-2-methylphenoxy)acetic acid} is one, it is available in solution (bottles to big barrels) ~750g/L as the dimethylamine salt.
Now, the hydrolysis product of this is of course the 4-chloro-2-methylphenol, has anyone got any ideas on how to get from the hydrolysis product to
either the p-Toluquinone or p-Toluquinol, because if so, the Fries Rearrangement of the 2,5-diacetoxytoluene is reported to give good yields of the 4-methyl-2,5-dihydroxyacetophenone, which might be
useful.
quam temere in nosmet legem sancimus iniquam
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That MCPA does not hydrolyse. It is not an ester, it is an ether. It can thus be utmost cleaved to 4-chloro-2-methylphenol or possibly oxidized
directly to p-toluquinone. Direct cleavage of phenoxyacetic acids seems to be very difficult and most literature examples use the roundabout methods
(typically the Curtius rearrangement) to obtain the phenol. The p-chloro substituent certainly does not ease the oxidation of that cresol and thus
limits the oxidation methods to only a few (for example, potassium nitrosodisulfonate). But then again, even if for some reason you can not obtain o- or m-cresol, toluene can be oxidized directly to
p-toluquinone. Actually, by electrooxidation this can be achieved without using any fancy reagents. Certainly way more easy than anything you could
come up with MCPA.
However, that herbicide is an interesting starting material for a number of experiments and if you can get it and isolate it in pure form, then do so
an use it for practice in synthesis: esterifications, amide formations, heterocyclic chemistry, electrophilic aromatic substitutions, oxidation of the
side chain, etc.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
Well there are several alternatives - there are oxidative, dechlorination procedures that will give the p-tolulquinone - I can't find the reference
for the direct oxidation of Toluene to the p-Toluquinone (could you be so kind as to direct me to one please?)...
Anyway, here are two papers which are fairly mild, giving a mix of products, but which oxidize p-chlorophenols (and ortho-substitued variants of the
same 2-methyl-4-chlorophenol is descried).
PS I am intrigued, are there any particular reactions why you'd suggest utilizing the free 2-chloro-4-methylphenol/2-chloro-4-methylphenoxyaxcetic
acid/
Attachment: Bates.Sammes.Thomson.Synthesis.of.the.C.Glycoside.Fragment.of.Nogalamycin.and.Nogalamycin.Precurors.pdf (1.3MB)
This file has been downloaded 944 times
Attachment: Ukrainczyk.McBride.Oxidation.and.Dechlorination.of.Chlorophenols.in.Dilute.Aqueous.Suspensions.of.Manganese.Oxides.React (1.1MB)
This file has been downloaded 1424 times
quam temere in nosmet legem sancimus iniquam
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by un0me2  | | I can't find the reference for the direct oxidation of Toluene to the p-Toluquinone (could you be so kind as to direct me to one please?)...
|
Nearly all direct oxidations of toluene to p-toluoquinone use H2O2 in the presence of some metal oxide catalyst, but a complex mixture is obtained of
which p-toluoquinone is not necessarily the main product (they all look pretty much useless and I did not even bother checking beyond the graphical CA
abstracts). The most simple and amateur friendly synthesis is the anodic oxidation of toluene in methanol. I don’t remember if there is any example
specifically on toluene, but the method seems pretty general and tolerates alkyl, alkoxy, aryl and halogen substituents. The oxidation gives a
p-quinone acetal which upon mild hydrolysis gives the corresponding p-quinone: US4046652, US4082809, US5098531, Synthesis (1979) 603-605
& 605-607.
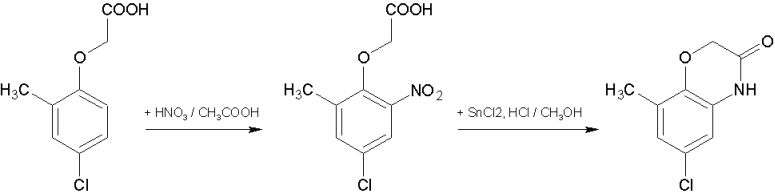
| Quote: | | PS I am intrigued, are there any particular reactions why you'd suggest utilizing the free 2-chloro-4-methylphenol/2-chloro-4-methylphenoxyaxcetic
acid |
I don't know what chemicals and equipment you have, so I can't just plan experiments for you. There are numerous things you can do with such a
substrate. For example, you can nitrate it and then reductively cyclisize to the corresponding benzoxazinone. This would include amateur friendly
transformations and would have good value for learning new chemistry and practice. It shouldn’t be difficult to obtain all the required materials,
also because both steps can use alternative reagents and conditions. The nitration could possibly work with K/Na/NH4 nitrate with an equivalent of
H2SO4 added in case you don't have any HNO3. For the reductive cyclocondensation there are also other conditions described in the literature and
patent examples, of which the simplest is Fe/CH3COOH or FeSO4 in the presence of NaOH(aq) or NH3(aq). Both products are solids, therefore suitable for
simple isolation, purification by recrystallization and characterization via mp. Though unfortunately both are new compounds not yet mentioned in the
literature. If you want to try something like this or need references, let me know.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
un0me2
aliced25 sock puppet
  
Posts: 205
Registered: 3-2-2010
Member Is Offline
Mood: No Mood
|
|
I actually have next to no equipment at the present time, between the clean-up (post accident) and all the rest, I'm embarrassingly bereft of the
capacity to do anything much at all. I'm slowly going to build it all back up, but
time & money are both tight. That said, the hydrolysis of a salt shouldn't be too hard, and I am looking to build up so I can show the kids some
basic chemistry.... I'm slowly going to build it all back up, but
time & money are both tight. That said, the hydrolysis of a salt shouldn't be too hard, and I am looking to build up so I can show the kids some
basic chemistry....
The reason I was asking was in case you had something specific in mind, at the present time I am looking at extracting the 2 n3 acids from fish oil
(the ethanolamide of each are anandamide analogues). As the two n3 polyunsaturated acids reportedly make up the bulk of the lipids, the urea adduct
should suffice (I have a Russian paper on the formation of the ethanolamide from the ethyl ester of each).
Anyway, back on topic - the review on Fremy's Radical suggests that it may work (page 2/18, top-right corner, of the attached paper):
When the position para to the hydroxyl group in 2 is unsubstituted (R = H), p-benzoquinones (3) are formed (pathway a). If
the position para to the hydroxyl group is substituted (R = OR, alkyl), oxidation leads to the formation of o-benzoquinones (4) (pathway b). One
exception has been reported, namely when R = C1. In this case, the oxidation proceeds, via pathway a, to form p-benzoquinones with the loss of
chlorine.
I'll have a look around and see if there is another oxidant which will do the job. I'm pushing to try and find a persulfate procedure (it is rather
easier to buy persulfate).
PS Yes, with regard to the direct oxidation of toluene to toluquinone, I couldn't seem to find anything that appealed - in terms of either yield or
isolation, so I was wondering...
Attachment: Zimmer.Lankin.Horgan.Oxidations.with.Potassium.Nitrodisulfonate.Fremys.Radical.The.Tueber.Reaction.pdf (1.3MB)
This file has been downloaded 1539 times
quam temere in nosmet legem sancimus iniquam
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by Nicodem  |
| Quote: | | PS I am intrigued, are there any particular reactions why you'd suggest utilizing the free 2-chloro-4-methylphenol/2-chloro-4-methylphenoxyaxcetic
acid |
I don't know what chemicals and equipment you have, so I can't just plan experiments for you. There are numerous things you can do with such a
substrate. For example, you can nitrate it and then reductively cyclisize to the corresponding benzoxazinone. This would include amateur friendly
transformations and would have good value for learning new chemistry and practice. It shouldn’t be difficult to obtain all the required materials,
also because both steps can use alternative reagents and conditions. The nitration could possibly work with K/Na/NH4 nitrate with an equivalent of
H2SO4 added in case you don't have any HNO3. For the reductive cyclocondensation there are also other conditions described in the literature and
patent examples, of which the simplest is Fe/CH3COOH or FeSO4 in the presence of NaOH(aq) or NH3(aq). Both products are solids, therefore suitable for
simple isolation, purification by recrystallization and characterization via mp. Though unfortunately both are new compounds not yet mentioned in the
literature. If you want to try something like this or need references, let me know. |
I don't think that wouldn't work, the nitration, in your scheme above, ought to go mainly para to the methyl group.
|
|
|
cheeseandbaloney
Harmless

Posts: 21
Registered: 4-4-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sandmeyer  | Quote: Originally posted by Nicodem  |
| Quote: | | PS I am intrigued, are there any particular reactions why you'd suggest utilizing the free 2-chloro-4-methylphenol/2-chloro-4-methylphenoxyaxcetic
acid |
I don't know what chemicals and equipment you have, so I can't just plan experiments for you. There are numerous things you can do with such a
substrate. For example, you can nitrate it and then reductively cyclisize to the corresponding benzoxazinone. This would include amateur friendly
transformations and would have good value for learning new chemistry and practice. It shouldn’t be difficult to obtain all the required materials,
also because both steps can use alternative reagents and conditions. The nitration could possibly work with K/Na/NH4 nitrate with an equivalent of
H2SO4 added in case you don't have any HNO3. For the reductive cyclocondensation there are also other conditions described in the literature and
patent examples, of which the simplest is Fe/CH3COOH or FeSO4 in the presence of NaOH(aq) or NH3(aq). Both products are solids, therefore suitable for
simple isolation, purification by recrystallization and characterization via mp. Though unfortunately both are new compounds not yet mentioned in the
literature. If you want to try something like this or need references, let me know. |
I don't think that wouldn't work, the nitration, in your scheme above, ought to go mainly para to the methyl group. |
Isn't the O the most electron donating (overtakes induction from -Cl and donation from methyl) which would put the electron density ortho to the ether
group? Or am I just overlooking the bulky sterics?
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Yo, cheeseandbaloney,  once one ortho position is substituted it is difficult
to for example nitrate the remaining one. Exceptions being for example phenols (highly activated for EAS) and for R-O-Ar directed ortho-metallation
(not for nitration though) which proceeds by a different mechanism and is of course always selective for ortho position. Take piperonal for example,
if we nitrate it we'll get nitration ortho to formyl and meta to alkyloxy, which looks weird if we consider that formyl group is deactivating,
slightly meta directing and that alkyloxy is ortho/para director and so, logically, the nitro should go meta to formyl and ortho to the methylenedioxy
ring -- but it dosen't. Why? That I would also like to know -- hey Arrhenius, any ideas? once one ortho position is substituted it is difficult
to for example nitrate the remaining one. Exceptions being for example phenols (highly activated for EAS) and for R-O-Ar directed ortho-metallation
(not for nitration though) which proceeds by a different mechanism and is of course always selective for ortho position. Take piperonal for example,
if we nitrate it we'll get nitration ortho to formyl and meta to alkyloxy, which looks weird if we consider that formyl group is deactivating,
slightly meta directing and that alkyloxy is ortho/para director and so, logically, the nitro should go meta to formyl and ortho to the methylenedioxy
ring -- but it dosen't. Why? That I would also like to know -- hey Arrhenius, any ideas?  I chose piperonal as an example since methylenedioxy ring, being conformationally restricted, pose minimal steric effects, in fact, if
anything is bulky in piperonal it is the formyl group. I chose piperonal as an example since methylenedioxy ring, being conformationally restricted, pose minimal steric effects, in fact, if
anything is bulky in piperonal it is the formyl group.
[Edited on 10-9-2010 by Sandmeyer]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sandmeyer  | Yo, cheeseandbaloney,  once one ortho position is substituted it is difficult
to for example nitrate the remaining one. Exceptions being for example phenols (highly activated for EAS) and for R-O-Ar directed ortho-metallation
(not for nitration though) which proceeds by a different mechanism and is of course always selective for ortho position. once one ortho position is substituted it is difficult
to for example nitrate the remaining one. Exceptions being for example phenols (highly activated for EAS) and for R-O-Ar directed ortho-metallation
(not for nitration though) which proceeds by a different mechanism and is of course always selective for ortho position. |
It is not difficult to nitrate (or substitute with other electrophilies) 2,4-substituted 1-activated substrates in the sense that the position 6 is
deactivated. It is difficult because of the competing ipso attacks on one of the occupied positions (usually at the position 4). The electrophile will
not attack a less activated site, as for example para to methyl in the case of MCPA, but instead it will attack the ortho position in regard to the
-OCH2COOH and also the ipso position of the chlorine group. The ratio depends on the electrophile, reaction media and substrate, but it is practically
always in favour of the ortho electrophilic substitution. For an example of nitration of p-chlorophenoxyacetic acid, yielding a product of ipso attack
(on the chloro position) as the minor product, see DOI:10.1139/v89-224.
To get an idea of how activating the -OCH2COOH group is, you can check DOI: 10.1021/ja00251a032 where nitrating p-methoxyphenoxyacetic acid gave a
70:30 ratio of isomers (ortho to MeO vs. ortho to -OCH2COOH respectively). The -OCH2COOH group is therefore only slightly less activating for EAS in
comparison to the methoxy and way more activating than a methyl group. It is thus hard to imagine a situation where the methyl group would prevail
over the -OCH2COOH group in dictating the position of the electrophilic attack.
| Quote: | Take piperonal for example, if we nitrate it we'll get nitration ortho to formyl and meta to alkyloxy, which looks weird if we consider that formyl
group is deactivating, slightly meta directing and that alkyloxy is ortho/para director and so, logically, the nitro should go meta to formyl and
ortho to the methylenedioxy ring -- but it dosen't. Why? That I would also like to know -- hey Arrhenius, any ideas?  I chose piperonal as an example since methylenedioxy ring, being conformationally
restricted, pose minimal steric effects, in fact, if anything is bulky in piperonal it is the formyl group. I chose piperonal as an example since methylenedioxy ring, being conformationally
restricted, pose minimal steric effects, in fact, if anything is bulky in piperonal it is the formyl group. |
Piperonal is not a very good example, because where you have two equally directing alkoxy groups for EAS and one has its para site blocked by
substitution (-CHO in piperonal) then its other equally activated para site is preferred, rather than any of their two ortho sites (perhaps due to the
strong negative inductive effect of the alkoxy groups and sometimes also due to sterics where applicable). But all thus also depends on the nature of
the electrophile. Nitrations of alkoxybenzenes are relatively selective, at least compared, for example to chlorinations, which tend to give a lousy
selectivity. For example, in the above cited JACS paper there is an example of 3,4-dimethoxyphenylacetonitrile nitration and there was a 100%
selectivity for the 4,5-dimethoxy-2-nitrophenylacetonitrile (in this substrate there is no -CHO group and the -CH2CN group is only very slightly
deactivating, nearly neutral).
Perhaps a more appropriate substrate to show the paradox that you wanted to show is the case of EAS on 2,5-dimethoxybenzaldehyde. It has bothered me
for quite some time why the bromination of this substrate gives 4-bromo-2,5-dimethoxybenzaldehyde. It was only when I draw the resonances for each of
the MeO donating an electron pair in the Pi system that I figured out the probable cause. The resonance structures resulting from the 2-MeO
electrodonation are all "neutralized" by the push-pull resonance with the -CHO group which is the thermodynamically most stable resonance state. The
less stable resonance state being the one with negative charge on position 5 and then the other two. The -CHO group interferes with the resonance
structures too strongly to allow the 2-MeO group dictating the electrophilic attack. Only the resonance structures resulting from the electrodonation
from the meta-MeO group are stable enough to promote the EAS at the position 4 and 6 (being free from the push-pull interference with -CHO). The
position 4 is favoured over 6 probably due to steric reasons (position 6 is hindered by a double ortho substitution which is usually quite
detrimental) and perhaps to a minor extent also due to the inductive effect of the -CHO group. At least that is how I explained the unusual
regioselectivity to myself. The same effect can explain also the regioselectivity of piperonal and veratraldehyde nitration, as well as lots of
related cases. However, with EAS reactions on piperonal and veratraldehyde this is not necessarily the only explanation, as demonstrated above.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Actually, I neglected something extremely obvious -- the other, meta alkyloxy group and considered only the alkyloxy para to the formyl and formyl
itself -- crazy *blush*! The formyl withdraws the electrons from the alkyloxy group para to it, the one that is positioned meta to the formyl does not
suffer from this electron withdrawal as much, in effect it is able to use those electrons to direct the incoming electrophile para and give
6-nitropiperonal.
Quote: Originally posted by Nicodem  |
The electrophile will not attack a less activated site, as for example para to methyl in the case of MCPA, but instead it will attack the ortho
position in regard to the -OCH2COOH and also the ipso position of the chlorine group. |
In any case, I don't think that the reaction you suggest will be selective. The literature claims that the nitro group indeed goes para to the methyl,
to what extent I can't say (don't have the paper), someone who cares could actually run the reaction, maybe those authors made an error, who knows.
[Edited on 14-9-2010 by Sandmeyer]
|
|
|
Anders2
Banned
Posts: 39
Registered: 4-9-2010
Member Is Offline
Mood: No Mood
|
|
Treat MCPA with a strong base at high temperatures. Chlorobenzene can be converted to phenol in this manner. The base will also hydrolyze off your
--OCH2COOH group, since phenol is somewhat acidic.
Then lightly acidify the salt that gets formed to free your desired compound.
You could perhaps react your compound with chlorine, before doing anything of the above, this would chlorinate that group, forming
--OCHClCOOH. This would be more vulnerable to hydrolyzing off in the presence of NaOH, but you would not want this to be heated with a strong base, as
some of it would then convert into --CH2OH.
[Edited on 16-9-2010 by Anders2]
[Edited on 16-9-2010 by Anders2]
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by Nicodem  |
It is not difficult to nitrate (or substitute with other electrophilies) 2,4-substituted 1-activated substrates in the sense that the position 6 is
deactivated. It is difficult because of the competing ipso attacks on one of the occupied positions (usually at the position 4). The electrophile will
not attack a less activated site, as for example para to methyl in the case of MCPA, but instead it will attack the ortho position in regard to the
-OCH2COOH and also the ipso position of the chlorine group. |
IMO this is not an obvious example although you seem to be quite confident.  But
you could follow your own advice and do a litterature search (or run the experiment)? Well, turns out that there are actually two papers [1,2]
describing the nitration of MCPA and both report the product resulting from nitro going para to the methyl while no ref (at least none that I could
find) supports your claim. But you are always welcome to conduct the experiment and validate what's published. Unfortunately I'm unable to get those
papers... But
you could follow your own advice and do a litterature search (or run the experiment)? Well, turns out that there are actually two papers [1,2]
describing the nitration of MCPA and both report the product resulting from nitro going para to the methyl while no ref (at least none that I could
find) supports your claim. But you are always welcome to conduct the experiment and validate what's published. Unfortunately I'm unable to get those
papers...
[1.] Faulkner, J. K.; Woodcock, D. Fungal detoxication. VII. Metabolism of 2,4-dichlorophenoxyacetic and 4-chloro-2-methylphenoxyacetic acids by
Aspergillus niger. Journal of the Chemical Society (1965), 1187-91
[2.] Eckstein, Zygmunt; Dyszer, Elzbieta; Niedzwialowska, Teresa. Nitration of 2-methyl- and 2-ethyl-4-chlorophenoxyacetic acid esters. I. Synthesis
of 2,4,5-trialkyl derivatives. Roczniki Chemii (1964), 38(1), 51-9.
Quote: Originally posted by Nicodem  | | The -OCH2COOH group is therefore only slightly less activating for EAS in comparison to the methoxy and way more activating than a methyl group. It is
thus hard to imagine a situation where the methyl group would prevail over the -OCH2COOH group in dictating the position of the electrophilic attack.
|
Why is it hard to imagine? -Cl is deactivating by induction, but weakly activating by mezomerism, the deactivating effect of -Cl can be imagined to be
off-set by the electron donation coming from the para alkyloxy group, this leaves -Cl weakly ortho activating. Methyl group is activating,
para-directing, so we are left with two activating effects in favour for the para-methyl substitution and one mesomerically activating effect for
ortho-alkyloxy. For those reasons I can see the nitration going para to methyl and this is also supported by the litterature.
Actually, this example has some similarities to piperonal example, in that it has two activating and one deacivating substituent in a same
substitution pattern. Since electron densities are related to the NMR shifts we can take a look at the measured 1HNMR shifts (from the literature) of
piperonal and MCPA and compare them, see the below picture. The difference between the NMR shift-values at the relevant positions is almost identical
(0.49 vs. 0.48) and so we could expect the nitration of MCPA to proceed according to the similar pattern as nitration of piperonal. All IMO of
course...
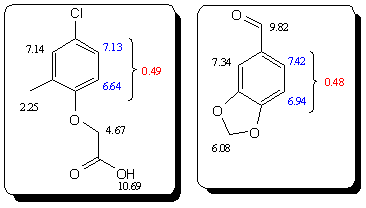
[Edited on 3-10-2010 by Sandmeyer]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sandmeyer  | IMO this is not an obvious example although you seem to be quite confident.  But
you could follow your own advice and do a litterature search (or run the experiment)? Well, turns out that there are actually two papers [1,2]
describing the nitration of MCPA and both report the product resulting from nitro going para to the methyl while no ref (at least none that I could
find) supports your claim. But you are always welcome to conduct the experiment and validate what's published. Unfortunately I'm unable to get those
papers... But
you could follow your own advice and do a litterature search (or run the experiment)? Well, turns out that there are actually two papers [1,2]
describing the nitration of MCPA and both report the product resulting from nitro going para to the methyl while no ref (at least none that I could
find) supports your claim. But you are always welcome to conduct the experiment and validate what's published. Unfortunately I'm unable to get those
papers... |
Unfortunately I do not have any MCPA, but I did check the JCS paper (attached). It indeed claims the 5-nitro regioisomer as the major product, but
caution should be used in interpreting that result. They used the method that looks suspiciously similar to the traditional
sulfonation-nitration-desulfonation strategy. This is a method where the substrate is dissolved in conc. H2SO4 to allow the most activated position
being sulfonated and then the nitrating mixture is added. The sulfonated substrate gets nitrated and since the nitro group is highly electron
withdrawing the product desulfonates. Sometimes this strategy can be used to block up to two most activated positions, particularly if using oleum as
the reaction media (in this case desulfonation occurs only after dilution with water). For example, did you know that you can even make
2-nitroresorcinol this way by nitrating resorcinol? The authors did not came up themselves with the idea of using H2SO4 as the reaction media, but
refer the origin of the method to the synthesis of 2,4-dichloro-5-nitrophenoxyacetic acid described in another JCS paper (DOI: 10.1039/JR9540000565,
attached). However, what is most interesting, these other authors refer to other two papers where the nitration also gave the 5-nitro isomer. I
checked one of them (DOI: 10.1021/jo01157a026) and this one was nitration in plain HNO3 (without H2SO4), so at least in this case we can be sure the
isomer formed is not due to sulfonation-nitration-desulfonation methodology. This is highly unusual because electrophilic aromatic substitutions on
2,4-dichloroanisoles occur at the position 6, except that I could not find any example for the nitrations on SciFinder. Could the nitration be an
exception? The same situation is with 2-alkyl-4-chloroanisols where the electrophilic substitutions occurs on the position 6, yet again I found no
examples for nitration. I have no satisfactory explanation for this, but I find your explanation unsatisfactory as well. How is the deactivating
effect of Cl supposed to be off-set by the -OCH2COOH when both are inductively -I and the chlorine is not on the correct position to gain electrons
from the oxygen's lone electron pairs by resonance? There must be a better explanation. Possibly the bulkier nitronium electrophile refuses to
substitute at the most reactive site and goes to the second one, thus giving the 5-nitro isomer? Doesn't sound very likely, but in the absence of
examples of other electrophilic substitutions on MCPA or 2,4-dichlorophenoxyacetic acid it is not easy to say anything particularly reasonable. There
is also to take into consideration that the nitration of 4-chlorophenoxyacetic acid gives the 2-nitro isomer.
By the way, the 1H NMR chemical shifts support the hypothesis that the electrophilic attack in MCPA would occur at the position 6, which apparently it
does not. The same goes for piperonal. Obviously trying to predict the most reactive position this way is of no use on such substrates. I guess that
is why it is not used in general as well.
Attachment: JR9650001187.pdf (142kB)
This file has been downloaded 921 times
Attachment: JR9540000565.pdf (98kB)
This file has been downloaded 914 times

…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Quote: Originally posted by Nicodem  |
By the way, the 1H NMR chemical shifts support the hypothesis that the electrophilic attack in MCPA would occur at the position 6, which apparently it
does not. The same goes for piperonal. Obviously trying to predict the most reactive position this way is of no use on such substrates. I guess that
is why it is not used in general as well.
|
I am aware that the lowest shift value corresponds to highest electron density. Was I attempting to predict the most reactive region on MCPA (or
piperonal) by looking at the chemical 1HNMR shifts within those isolated molecules? No, the interest was to predict a pattern and not to draw
conclusions from isolated values.
If we know that piperonal and MCPA have the same substitution pattern, and we know that piperonal has a certain 1HNMR shift pattern and this pattern
directs the nitraton on position 6, and if MCPA follows essentially the same 1HNMR shift pattern as piperonal why wouldn't MCPA also direct nitration
to corresponding position?
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Ok, then sorry if I was unclear. What I meen is, when we consider MCPA there is one ortho-activating -Cl substituent, one para-activating methyl
substituent and one ortho-activating alkyloxy substituent. Due to the synergy between two activating effects towards the pos. para to methyl the
nitration should go there rather than ortho to alkyloxy group. Maybe this is after all best explained with good old reasonance structures. Path a.)
represents the substitution para to the methyl, path b.) represents the substitution ortho to alkyloxy, both are similar except one difference, path
a.) has a tertiary carbotion (blue Weeland intermediate below) as one resonance structure, path b.) has two secondary carbocations and since tertiary
carbocations are more stable than secondary the nitration should proceed via path a.). But I don't know how the effect of better p-orbital overlap of
oxygen vs. -Cl with the pi system of the phenyl ring competes with the stabilizing effect I mentioned above (tertiary vs. secondary carbocation) hence
I tried to find some relationship between experimental NMR shift-values and reactivity as I explained above as NMR shifts are related to electronic
effects.
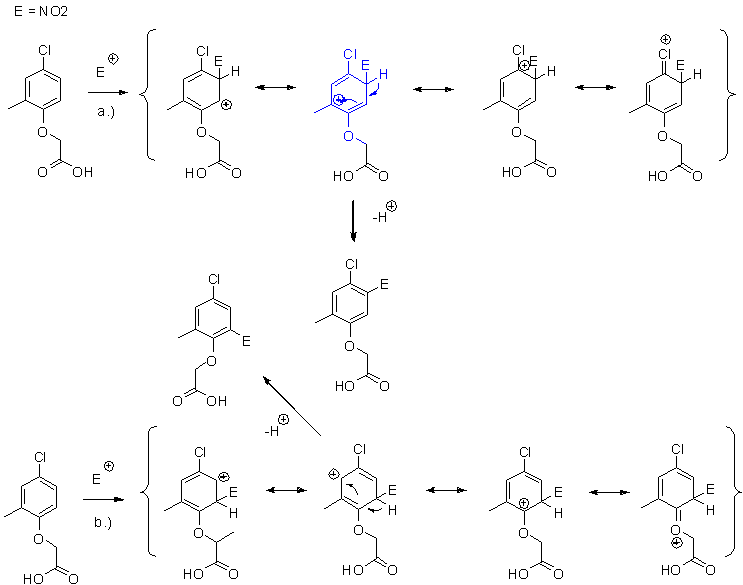
[Edited on 4-10-2010 by Sandmeyer]
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
maybe copper mediated nucleophilic substitution of chlorine to methoxy or hydroxy would work well on this substrate, because phenoxyacetate is
possibly a weak chelating agent and can increase a concentration of catalytically active copper in basic solution by forming a chelate. This is a
proposal anyway. Another one is that chelated phenoxy oxygen atom would partially lose its +M effect and develop some -I, but still not sufficient to
become a EWG group out of donating group. But if this effect can somehow cooperate with a mechanism of copper mediated substitution, then the
substrate would be more prone to nucl.substitution. And after that(substitution to OH) oxidation to quinone would proceed much easier.
If nucleophilic substitution does not work, you may make an amide of that substance and hoffmann rearrangement with further hydrolysis would relieve a
phenolic OH group and the product can be oxidized to quinone then
[Edited on 5-10-2010 by Ebao-lu]
[Edited on 5-10-2010 by Ebao-lu]
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
rearrangement for better satisfaction
Quote: Originally posted by Sandmeyer  |
But I don't know how the effect of better p-orbital overlap of oxygen vs. -Cl with the pi system of the phenyl ring competes with the stabilizing
effect I mentioned above (tertiary vs. secondary carbocation) hence I tried to find some relationship between experimental NMR shift-values and
reactivity as I explained above as NMR shifts are related to electronic effects.
[Edited on 4-10-2010 by Sandmeyer] |
That more efficient orbital overlap (oxygen vs. chlorine) can not be ignored, after all -Cl isn't an alkyloxy, but then if I satisfy this fact by
letting the nitraton go ortho to the alkyloxy I end up on path b.) (picture above) and lose the tert. carbocation stability.
Obviously a mechanism has to be proposed that can allow contribution from both stabilizing effect of tert. carbocation (path a. in the picture above)
and the effect of more efficient p-orbital overlap from oxygen (vs. that of -Cl) onto the pi-system of the ring. But in order to satisfy the frontier
orbitals we don't have to let the electrophile only go ortho to the alkyloxy, we might as well let it go para. That gives us the weird-looking Weeland
intermediate (red) (see picture below) and a little trick in form of a 1,2-shift sets the nitro in a position where we can also take the advantage of
tert. carbocation stabilization. Orbitals happy + carbocation happy = SWiM happy...
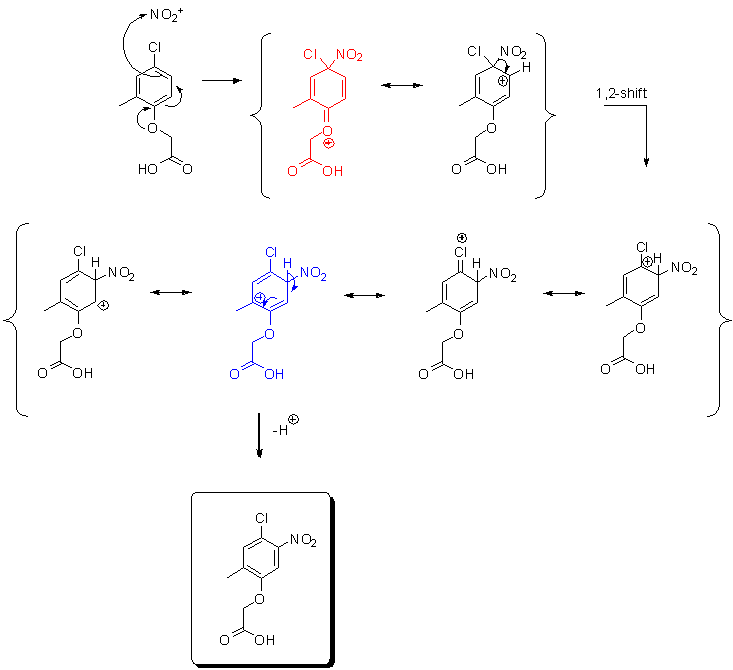
[Edited on 8-10-2010 by Sandmeyer]
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
I think thats mostly because of protone "chelating". Carboxylic acids in conc H2SO4 are known to be protonated, at least for instance benzaldehyde is
soluble in conc H2SO4 fot that reason. And in this case there is chelated proton, like a "bridge" between carboxyl group and phenoxy oxygen, and this
cation is much more stable, increasing the percent of protonated carboxylic acid groups(as you may know, the most strong organic bases are some of
aromatic diamines that also form a proton "bridge"). In this chelate, the phenoxy oxygen partually loses its +M effect and develops -I, becoming
partually a deactivating group and making ortho-substitution not that beneficial. So, nitro group goes to meta position.. Besides, there could be an
impact of electrostatic repulsion between NO2+ and that chelated proton.
The difference between 4-chloro, 2-Me(Cl)-4-chlorophenoxyacetic nitration products may result from additional stabilization of 5-product's proton
sigma complex by +I/ +M effects of methyl/chlorine atoms in position 2. So, this impact together with oxygen's lost electrons in chelating is
sufficient for orienting substituent into position 5 rather then 6, while only the oxygen electron loss is not. In other words, in this chelate oxygen
still provides some activation of o,p-positions, but it is deminished. And 2 weak orientants together, like Cl,Cl, or Me,Cl, can provide a better
activation, while only one chlorine can not
Other hypothetical reason that causes this difference between 4-substituted and 2,4-disubstituted reaction products could be that substituent
2(especially chlorine) is quite hydrophobic. It makes more problems for hydrosulfate, sulfuric acid, and other polar molecules to come close to
chelate's protone and form a surrounding then in the case where there is no 2 substituent. The less there is polar surrounding, the more phenoxy
oxygen atom would lose its +M effect and develop -I. Besides, this hydrophobic substituent can also prevent another kind of stabilization - polar
molecules would not come close to second proton, which is not chelated. This also makes more electrons to be withdrawn from phenoxy oxygen.
[Edited on 8-10-2010 by Ebao-lu]
|
|
|
|