| Pages:
1
2
3 |
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Exotic Oxidizers
Anyone done any sugar burns, tests with Al, Mg, or S with these oxidants.
Sodium Bismuthate
Strontium Peroxide
Sodium Percarbonate
Nitrites
Ferrates
Sodium Chlorite
Dichromates
Calcium Hypochlorite
Complex salts:
Tetraminecopper(II) chlorate
Tetraminecopper(II) persulfate
Tetraminenickel(II) persulfate
Organics:
meta-Chloroperoxybenzoic acid
|
|
|
Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
Amonium dichromate is bad idea  I had used Potasium persulfate, potasium
superoxide and sodium peroxide. I had something i maded maybe its Tetraminecopper(II) persulfate no sure now. I had used Potasium persulfate, potasium
superoxide and sodium peroxide. I had something i maded maybe its Tetraminecopper(II) persulfate no sure now.
|
|
|
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I know about Ammonium Dichromate. It is used in the famous chemical volcano demonstrations where it decomposes into Cr(III) oxide
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Strontium peroxide has been used in bullet/shell tracers and
igniters, e.g.,
Tracer Red R256
Mc Page 279 Pep 12(1)
Magnesium 50 100
Strontium Nitrate
Strontium Peroxide
Calcium Resinate Type 1
Calcium Resinate Type 2
Magnesium
Igniter R20C
MC 282 AMCP 706-184
Strontium Peroxide
Calcium Resinate
Barium Peroxide
Lead Dioxide
Magnesium
Tetraaminecopper chlorate. Anhydrous it is a primary explosive.
Hydrated — long ago it use was suggested by the French amateur pyrotechnist Chertier.
The American Druggist An Illustrated Monthly Journal of Pharmacy, Chemistry and
Materia Medica
FRED'K A. CASTLE, M.D., EDITOR
VOL. XVIII.
WILLIAM WOOD & COMPANY
PUBLISHERS
56 & 58 LAFAYETTE PLACE, NEW YORK
1889
June 1889
Chlorate of Potassium as an Explosive.
We learn from a report of Her Majesty's Inspectors of Explosives
that Mr. Dupre, the chemist to this department,
last year had to investigate an accident in Pain's fireworks factory arising from the
explosion of colored stars. The results are of interest as corroborating previous
observations regarding the highly sensitive nature to percussion and friction of
chlorate mixtures, particularly at slightly elevated temperatures. The chemicals
employed in the manufacture of the stars were found to be chlorates of barium and
potassium, nitrate of strontium, shellac, coal, and lamp-black. Lamp-black is liable
to contain an appreciable quantity of free sulphuric acid, but there was none in this
case. It was found, however, that one of the ingredients (Chertier's copper) of one of
the stars was distinctly acid, and was the cause of the explosion. Chertier's copper is
a mixture of chlorate of potassium and sulphate of copper, which has been
moistened with ammonia and dried, when freshly made it is alkaline, but in time it
loses ammonia, becomes acid, and evolves chlorine compounds, owing to the
decomposition of the chlorate of potassium by the sulphate of copper. In other
words, Chertier's copper is liable to spontaneous decomposition, and the presence
of such a substance in a combustible or explosive mixture cannot but be highly dangerous.
For a complete discussion of Chertier's Copper see Brian Bush,
Chertier's Copper(s) Pyrotechnics Guild International, Inc.
Bulletin 129, May/June 2002.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Speaking of which...
Strontium peroxide was our red tracer & Barium peroxide was the Soviets Green tracer. There ARE various bullet formers and drilling rigs to put a
5.56 or a 7.62 and drill just enough to the butt end (don't use boat tails, they crowd the drill bit) and then press into it your tracer composition
(density needs to be high) & sealant (NC lacquer / Mg / meal powder: VERY tiny quantities). And they work VERY well. The resonates quoted
previously require exceedingly high pressure levels to retain the composition within the bullet. The sealant concept was used in one patent so as to
protect the compostion from any possiblity of falling back into the propellant (a very serious issue as pressur spikes could result in rupured cases).
The .45 ACP actually is the best because of the volume. When removing lead the drill has a "stop" so you have some degree of consistency. Then you
weigh and after you press your composition in and seal, you weigh again and formulate your load to the final weight which is not TOO far away from
your original. Most everything is loaded from the rear due to set-back from firing pressure & ignition dynamics which occur via natural propellant
heat.
The science of balancing the heat of propellant discharge & / or the use of special primer may also provide a large experimental pool of
information & testing to achieve desired effect.
Somewhere is the US patent for our .50BMG incendiary round. I don't know the history of it's production or implementation but it is exceedingly
difficult to do properly without plant-level production techniques. {I believe it's sealed WP, which unwraps & is exposed when the bullet strikes
home.} There is also an explosive round. The only patent I saw was for the 20mm & larger cannon; again, very difficult to emulate.
These tooling sets were commonly sold some years back. As in accordance to Fed statutes, tracer has few restrictions. Explosive rounds are another
story altogether. The biggest issue is that State law may be problematical and of course, there is a very real fire danger as with all pyrotechnics.
[Edited on 14-8-2010 by quicksilver]
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by mewrox99  | Anyone done any sugar burns, tests with Al, Mg, or S with these oxidants.
Sodium Bismuthate
Strontium Peroxide
Sodium Percarbonate
Nitrites
Ferrates
Sodium Chlorite
Dichromates
Calcium Hypochlorite
Complex salts:
Tetraminecopper(II) chlorate
Tetraminecopper(II) persulfate
Tetraminenickel(II) persulfate
Organics:
meta-Chloroperoxybenzoic acid
|
I tried some of these:
sodium bismuthate --> bright orange flash with Al, moderately easy to ignite
Strontium peroxide (I used barium peroxide, very similar) --> bright white flash with Al, moderately easty to ignite
Sodium percarbonate --> no real flash, simply decomposes on heating
Sodium nitrite --> no pyrotechnics at all, even with red phosphorus it does not burn
sodium chlorite --> exceedingly dangerous with many reductors, including aluminium powder. Very powerful flash reaction, but unstable and can
self-ignite!
potassium dichromate --> no pyrotechnics at all, simply melts. Even with red phosphorus it gives no burning reaction.
calcium hypochlorite --> similar to sodium chlorite, even more reactive and self-ignites with many reductors, especially if somewhat humid. With
red phosphorus and aluminium mixed in, this gives a self-igniting mix, which ignites a few minutes after preparation.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Woelen:
When you mention sodium chlorite as being a possible self ignition, is there special shipping/ storage agenda? OR is this in relation to exposure or
contact w/ fuel, etc
|
|
|
woelen
Super Administrator
        
Posts: 8082
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The self-ignition only is when it is mixed with certain reductors. Sodium chlorite itself (85%, the balance being NaCl) is perfectly stable on storage
and also can be shipped without problems. I purchased it as MMS/water purifyer from an eBay seller.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
Somewhere is the US patent for our .50BMG incendiary round. I don't know the history of it's production or implementation but it is exceedingly
difficult to do properly without plant-level production techniques. {I believe it's sealed WP, which unwraps & is exposed when the bullet strikes
home.} There is also an explosive round. The only patent I saw was for the 20mm & larger cannon; again, very difficult to emulate.
|
The .50 BMG Gov. surplus round I have use barium nitrate and
magnalium. Against a 3/8" steel plate at 100-yards yields a
puff of smoke. I heed someone to fire my rifle so I could watch
with field glasses.
I am up to my muzzle break in .50 BMG tracer, problem being
it has to go 200 or so yards before it will ignite and I am not
willing to let fly in my woodlot with it starting a fire or two. This
being the reason tracer ammo is illegal in California.
What you do be wanting is Raufoss ammo. Incendiary ammo, it
uses zirconium. This from Wiki-P
http://en.wikipedia.org/wiki/Raufoss_Mk_211
Make sure your sitting down when you check the price for the
.50 BMG stuff. They also make 30/06 which is a lot less money.
Run tracer for ammunition through Google.com/patents.
"Until we went to war with Germany our Army had known only the cartridge firing
hard-jacked lead bullet. But we entered a conflict in which several novel sorts of
small-arms projectiles were in familiar use; and it became necessary for us to
take up the manufacture of these strange missiles at once. These included such
special types as ....incendiary bullets for setting on fire observation balloons ....
"On of the first acts of the Ordnance Department was to send an officer to visit
the ammunition factories of France and England to study the methods of
manufacturing these special types of bullets..... Special machinery was required
to for the loading of tracer bullet and also for producing the incendiary projectile.
We adopted the British practice for both of these....
"An entirely different principle was used in the construction of the incendiary
bullet. This bullet was also was also incased in cupronickel; but the incendiary
chemical, which was phosphorus, was contained in a chamber in the nose of the
bullet in the nose of the bullet. [Not very good photo of a sectioned cartridge.] A
serrated plug held the phosphorus in its chamber, and behind this plug was a
solid plug of lead coming flush with base of the bullet and soldered thereto. On
one side of the missile was a hole drilled through the cupronickle into one of the
groves of the serrated plug. This hole was stopped by a special kind of solder.
The heat of friction developed in the infinitesimal space of time while the
projectile was passing through the gun barrel served the double purpose of
melting out the solder from the hole and igniting the phosphorus within the
chamber. Thereafter the centrifugal force of the revolving bullet whirled the
burning phosphorus out through the unplugged hole. Seen in the air the fire of
the phosphorus could not be descerned, but the burning chemical threw off
considerable smoke, so that the eye of the gunner could follow the
blue spiral to its mark. Our incendiary bullet had an effective range of 350 yards,
after which distance the phosphorus was burned out."
America's Munitions 1917-1918
Report of Benedict Crowell
The Assistant Secretary of War- Director of Munitions
Washington Government Printing Office 1919
I own an original copy - you can get yours from Google.com/books
IM-11 is listed for both incendiary and API bullets - 50% Mg/Al (50/50) 50%
Barium nitrate.
The current leader-of-the-pack is the Raufoss (also spelled Raufus) NM 140.
This from Jane's Ammunition Handbook 1994-95 --
Manufacturer: Raufoss Ammunisjonsfabrikker A/S [Norway].
Type: Multipurpose NM 140: The exterior of this round conforms to the standard
US M2 Ball, but the bullet is a design patented by Raufoss which incorporates
penetrative, incendiary and fragmentation effects. NM 140 will defeat 16 mm of
armour (Brinell 360) at 30 (sup)o at 400 m; after penetration it will explode and
produce about 20 effective fragments inside the target. There will also be a
shower of incendiary particles which are still effective 15 m behind the target
plate. No mechanical fuze is used because the need for detonators or sensitive
high explosives has been eliminated. Muzzle velocity 900 m/s, bullet weight 43 g.
You can find them for sale at the usual place. I am saving up my
money so I can buy one or two .50 BMG ones.
If you run Raufoss & Projectile though google.com/patents you will
find 27 patents, 3 677 181 I suspect is the one you want.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
These tooling sets were commonly sold some years back. As in accordance to Fed statutes, tracer has few restrictions. Explosive rounds are another
story altogether. The biggest issue is that State law may be problematical and of course, there is a very real fire danger as with all pyrotechnics.
|
Cut and paste and therefore some of the inmates here
think useless - early explosive bullets stuff attached.
Attachment: Pyro Explosive bullets.docx (28kB)
This file has been downloaded 1050 times
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
These tooling sets were commonly sold some years back. As in accordance to Fed statutes, tracer has few restrictions. Explosive rounds are another
story altogether. The biggest issue is that State law may be problematical and of course, there is a very real fire danger as with all pyrotechnics.
|
President Reagan as shot with exploding ammo.
Why didn't President Reagan allow Bill Brady to ride in
the Presidential Limo with the top down?
His head whistled.
Vincent J.M. Di Maio
Gunshot Wounds: Practical Aspects of Firearms,
Ballistics, and Forensic Techniques
Elsvier 1985
Exploding Ammunition
The 1970s saw the introduction of exploding ammunition for handguns.
Exploding ammunition dates back to the early nineteenth century and was
used in rifles in the American Civil War. Present-day exploding
ammunition intended for handguns has been manufactured in at least
three forms for centerfire cartridges and one form for rimfire cartridges.
Ammunition initially manufactured for centerfire weapons used ordinary
commercial semijacketed hollow-point ammunition in which the nose of
the bullet had been drilled out. Into this cavity was placed black powder
and a lead shot. The tip of the cavity was then sealed with a percussion
cap. Because of federal regulations regarding black powder, a second
form of exploding ammunition was introduced to replace the first. The
black powder was replaced by Pyrodex, a smokeless powder substitute
for black powder and a pistol primer replaced the percussion cap. The
third form of exploding ammunition is essentially the same, but no lead
shot is used.
Evaluation of a series of individuals shot with this ammunition reveals
that both the entrance wound and the wound tracks are indistinguishable
from wounds produced by similar nonexploding ammunition of the same
caliber.'° The fact that one is dealing with exploding ammunition may be
determinable only on x-ray, as often the primer cap and primer anvil may
be seen.
President Reagan was shot with .22 Long Rifle exploding ammunition.
This ammunition is constructed from ordinary commercially available .22
Long Rifle hollow-point ammunition. A hole is drilled in the tip of the bullet,
with insertion of an aluminum cylinder. The cylinder is filled with an
explosive mixture and sealed at its open end. The cylinder is inserted with
the sealed end toward the base of the bullet. Originally, RDX explosive
was used in the cylinder. but , this was replaced with lead azide.
Vincent J.M. Di Maio
Gunshot Wounds: Practical Aspects of Firearms,
Ballistics, and Forensic Techniques
People who found this book interesting also found interesting :—
Gary J Ordog Ed.
Mangement of Gunshot Wounds
Elsvier 1988
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
TRULY fascinating.
I never really had the $ to invest when you could get a Barret for a reasonable price (what IS reasonable?). A good friend has one that I had shot
quite a bit (he trusts me with his kids; so trusting me with his rifle is appropriate.....) Biggest issue is a getting a scope that will accommodate
it's potential.
The thing I think is possibly the MOST wonderful thing about a 50 is that it's big enough to experiment with ballistics, chemistry, physics, & a
wonderful combination of all of those sciences in a manner unavailable in any civilian tool. You're one lucky fellow.
I know this is really OT but I wish I had known you had one several months back as I had a chance to get an enormous amount of powder and brass for
pocket change. IF you ever want to, PM me & I'll see if it's still available.
[Edited on 14-8-2010 by quicksilver]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
The thing I think is possibly the MOST wonderful thing about a 50 is that it's big enough to experiment with ballistics, chemistry, physics, & a
wonderful combination of all of those sciences in a manner unavailable in any civilian tool. You're one lucky fellow. |
And self abuse. I forgot to put my ear protection on and fired
my bolt action 50 .... was like someone stuck knitting needles in
both ears!
Back in the late 50's? early 60's you could buy a semi-auto
20mm cannon for $1 500? (a lot of money then) and for a buck
a pop 20mm explosive ammo!
Some time ago the ATF declared anything with a rifled barrel
above 50 cal without sporting purpose a DD. Them that
owned them had to either turn them in destroy them. Dido
for .60 Boy's (there is controversy about the spelling)
British anti-tank rifle.
The WW II Yellow Peril had a 20mm rifle.
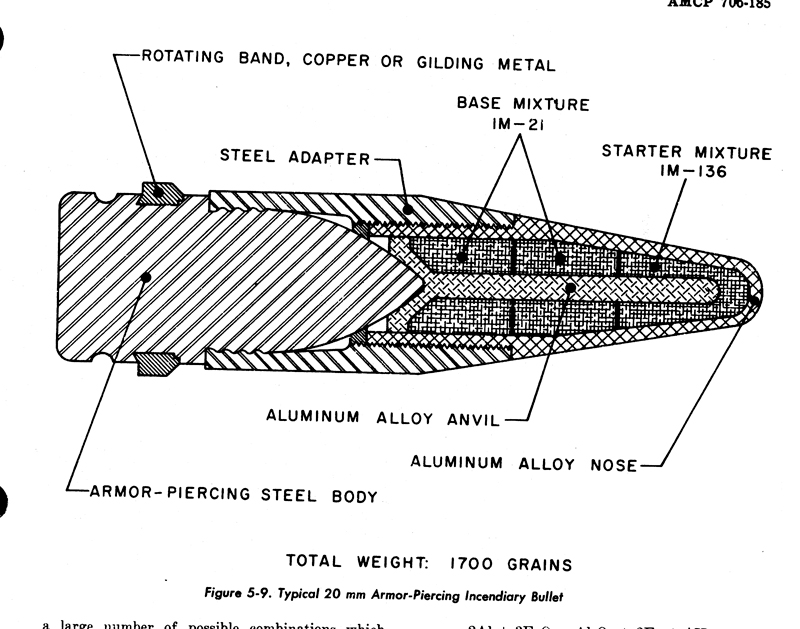
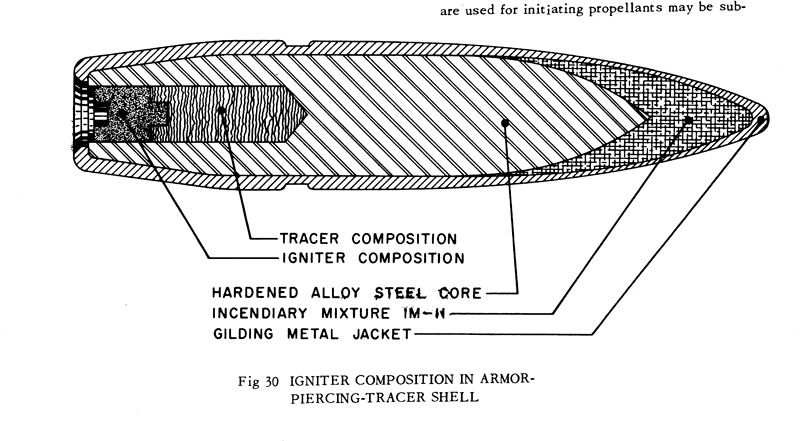
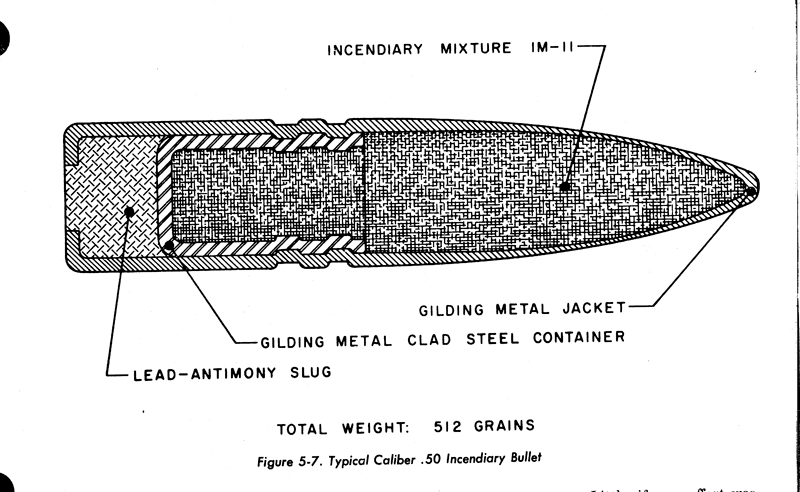
The 20mm from PATR-2700
.50 from AMCP 706-185
All are free DL's.
Somewhere in 2700 is a drawing of a .50 BMG explosive round.
Byda a while ago in the usual place someone was selling a
$12 000 lot of 50 BMG ammo. I calculate in a quard M2 [Ma-Duce ] it would last 4 minutes.
|
|
|
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Very interesting stuff.
Hey woelen have you tried any sugar or sulfur burns with those oxidants
|
|
|
Blasty
Hazard to Others
  
Posts: 107
Registered: 25-7-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  |
potassium dichromate --> no pyrotechnics at all, simply melts. Even with red phosphorus it gives no burning reaction. |
Chromates and dichromates don't seem to be good for such purposes on their own, but they do serve as "accelerators" in mixtures of chlorates or
perchlorates and some organic substances (ex: Berge's blasting powder, which burns way faster than plain chlorate-sugar mixtures.)
|
|
|
mewrox99
Hazard to Others
  
Posts: 321
Registered: 7-6-2010
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Has anyone tried a chlorite or a hypochlorite sugar burn.
And has anyone tried a bismuthate(VI) sugar burn
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Aahhhh balistics , very worthwhile hobby.
Standard .50 BMG 600 grain ball with a muzzle velocity of 2800 fps rates 10500 foot pounds.
The same cartridge loaded instead with a round of 180 grain .30 caliber boattail inside of a
30 grain sabo , will achieve a muzzle velocity of 4750 fps ! The normal rifle powder won't do ,
a load of faster burning pistol or shotgun powder will be needed to keep the pressure up.
The regular .50 cal round will range out to 2500 yards ( 1.4 miles ) , the sabo is good out
to 2 miles. Because of dispersion you'll be lucky to hit a car at that range if that's your target.
At closer range you can reliably pierce engine blocks & brick walls.
Barrets however command premium pricing
http://www.budsgunshop.com/catalog/product_info.php/products...
http://www.barrett.net
12 gauge shotguns are very under rated yet are the most versatile of small arms , 12 gauge
loaded with .50 caliber sabo rounds can approach the same performance level and for this
reason are unlawful in many districts.
Using bullet swaging equipment one can precision form small caliber shaped charge liner cans
to be explosive filled with an inertial base detonator , loaded into a shotgun shell " ring shot "
in which the forward part leaves the barrel as a whole unit projectile trailing streamers to act
as fins to keep it heading straight. http://www.corbins.com
.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
The stupid thing about the laws dealing with rifled barreled ballistic size is that the Federal powers that be did not consider the very expensive
English & German double rifles (drillings) that were 60-70cal "Nitro-express" commercial dangerous game weapons. Ahhhhh.....logic.
Enclosed are some DDNP experiments using oxidizers & the testing mechanism thereof. I believe too many people tossed off oxidizer additions as
methods of adulterants or mechanisms of "stretching a dollar" when utilized w/ primaries.
Attachment: DDNP-experiments with oxidizers_and_testing.pdf (997kB)
This file has been downloaded 903 times
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  | The stupid thing
about the laws dealing with rifled barreled ballistic size is that the
Federal powers that be did not consider the very expensive English &
German double rifles (drillings) that were 60-70cal "Nitro-express"
commercial dangerous game weapons. Ahhhhh.....logic. |
Au contraire - Because the .600 Nitro, .700 Nitro and
12ga shotguns w/ rifled barrels are considered to have
sporting use they are allowed to civilians.
A dbl bespoke (they all are) .700 Nitro isn't very expensive
it is extremely very expensive. Better bring your Black
American Express Card. The ammo is over a hundred US a
round, however, if it keeps a charging elephant from having sex with you....
Where are a elephants sex organs?
On his feet - if he steps on you - you are ......
From Wiki-P
While American Express does not publicly disclose the
requirements for getting a card, requirements have been
reported to include:
* An impeccable credit history.
* $250,000 in annual transactions with American Express
(around roughly $21,000 per month).
* A one-time card membership fee of $5,000. An annual fee of
$2,500 is also levied.
* Substantial net worth (undisclosed by American Express).
Because the Peoples Republic of California and France do not
allow private ownership of .50 BMG rifles there is now the .416
and another caliber that I cannot bring up my mind.
Physics
For every reaction there can be an unpleasant action.
"Around 1960, Fred Barnes (of Barns bullet fame and no
relation to the Frank Barns the originator of this book)
built himself a 475 A&M-chambered rifle, based upon a
sporterized Enfield action. ... that rifle weighed
no more than 8 pounds. ... his initial handloading
combined stiff charges of IMR 3031 behind his
600-grain bullets. His friends and a small group of
well-wishers went to an informal shooting range...
Fred sat down... [in a] sitting position. He took dead
aim at a the base of a small juniper tree, which was
tenuously hanging on at the top edge of a roadway
cutbank.
"When Fred pulled the trigger, everyone was watching
for the impact. The shot went low. The tree was summarily
uprooted! ... then as a group, they looked around to find
what Barn's reaction might be. There he was, located
several feet behind his original position lying on his back,
arms out stretched, holding his rifle above his head. Dust
from the muzzle blast and his ignoble recoil-induced slide
(he had absorbed well over 110 foot-pounds of energy) was
still stirring when Fred asked, matter-of-factly, "Anybody
want to buy a rifle?" He found no takers."
F Barnes.
Cartridges of the World
10th Edition
With an muzzle energy of 9 250 ft/lb fring a 400gr bullet at
3 227 ft/sec the .475 has a higher muzzle energy than the
.700 Nitro — 8 900 ft/lb 1 000gr @ 2 000 ft/sec.
Recoil is a function of bullet weight - speed and the amount
of powder burned which in large bore rifles can be considerable
Often overlooked is the velocity of recoil, in a light rifle such as
my .350 Rem 600 and Mosburgh 3" "12ga it gets your attention
with a heck of a kick.
My v/ heavy bolt-action .50 BMG with an efficient muzzle brake
doesn't kick it pushes.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Historical insanity note: The largest RIMFIRE CARTRIDGE IN HISTORY WAS THE "58 MILLER" with a 500gr bullet moving at over 1100fps. The last of them
may have been seen at the beginning of the 20th century like 1908-11
Last chlorate corrosive US mil ammo (I believe) was 1950's Frankfort Arsenal. - Issue was made more serious as the primer cups may have eventually
lost some of the materiel within the (brass) casing.
I really should have remembered the "sporting use clause' .... BUT, it may have been interjected or made clear during Clinton's Administration due to
a collection of problems in the wording of Fed statutes.
[Edited on 16-8-2010 by quicksilver]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
Last chlorate corrosive US mil ammo (I believe) was 1950's Frankfort Arsenal. - Issue was made more serious as the primer cups may have eventually
lost some of the materiel within the (brass) casing. |
I have some .50 BMG ammo, really hot ammo I need both hand
and a foot to open the bolt after firing it, so I don't use it.
Head stamp FNB 82 thus made in Belgium, 1982. I believe it is
corrosive primed.
Currently the big push is for lead free primers, run it through
Google.com/patents.
If you have the time read up on the US Army's attempts to
use red phosphorus in primers. They could never solve the
pure enough RP i.e., no spontaneous phosphorus pentoxide
yielding phosphoric acid. Several US patents.
Hatcher's Notebook
Julian S Hatcher Major General, U. S. Army, Retired
Stackpole
3rd. ed 1966
I strongly recommend this book.
[page numbers]
[353] Noncorrosive Primers
When the primer was definitely identified as the culprit behind
the gun corrosion trouble, it was only natural that there should
at once arise a great bustle of activity directed towards finding
a primer that would overcome this defect.
Let's go back a few years into primer history. For many years
after the first metallic cartridges came into use, all primers
had as one of their principal constituents a very sensitive
explosive called fulminate of mercury, and in addition, they had
more or less potassium chlorate. This mixture worked all right
with black powder, which deposits some percent of its weight in
the form of solids when it burns. This large amount of fouling
completely masked, diluted, and washed away the small amount of
solid material left by the primer combustion.
When smokeless powder came into use, the picture changed. The
explosion of the cartridge left the bore of the gun and the
inside of the cartridge clean and nearly free from fouling. The
material deposited by the primer combustion had a fine clear
place to land.
The first result was noted by the handloaders. In those days
everyone was more or less used to loading his own charges from
the powder horn and shot flask which hung by the old muzzle
loader that stood in the corner. When guns came into use that had
nice expensive brass cartridge cases to hold the charge, what was
more natural than to reload the empty ones; especially as the
black powder then in use was so flexible that no table of charges
was necessary.
As a result, handloading was almost universal in the days of
the early breech loader. When smokeless powder came into use, the
handloaders noted with dismay that the brass cases cracked after
being used only a very little. The cause of this cracking was
finally traced by the Ordnance Department to the Mercury
deposited from the fulminate used in the primer composition.
This was described by the Chief of Ordnance in his report for
the year 1897. The next year the Ordnance Department started
loading the service Krag cartridge with a non-mercuric primer.
When World War I came, the standard Mixture used by Frankford
Arsenal and known as FH-42, had the following- composition:
Sulphur .................. 21-97%
Potassium Chlorate ....... 47.20%
Antinomy Sulphide ........... 30-83%
This mixture superseded the former H-48, which contained ground
glass, thought by some shooters to injure the bore. This primer
[355] entirely eliminated the case cracking trouble, and was
really one of the most satisfactory ever used, until an
unexpected, and at the time, not understood, incident caused its
sudden abandonment.
The United States entered the First World War in April, 1917,
and the production of Frankford Arsenal was at once stepped up to
several times what it had ever been before. Then in May, trouble
began with misfires in the Frankford Service Ammunition. The
trouble was so serious that the entire ammunition plant was shut
down right when they needed its production worse than they had
ever needed it before. The best chemists and engineers available
were put to work to discover the cause of the misfires and the
remedy, but in the meantime months of production were being lost,
so that the Ordnance Department summarily ordered no more H-48
primers made, and directed Frankford to adopt and use forthwith
the Winchester Repeating Arms Company's primer, 35-NF which was then giving very satisfactory results.
This primer mixture had the following composition:
Potassium Chlorate ..... .53%
Antimony Sulphide....... .17%
Lead Sulpho-cyanide..... .25%
Tri-nitro-toluol (T.N.T.) .5%
After its adoption by Frankford Arsenal this primer became
known as F.A. No. 70, and has been used, with minor
modifications, ever since.
The trouble with the old sulphur primer was afterwards traced
to the overloading of the primer drying houses that occurred when
production was stepped up. The primers were loaded with the mix-
ture in a moist condition, and the loaded cups were then put in
the drying houses where steam heat was used to dry them out. When
the drying houses were overloaded, the presence of so many
primers in the warm room produced a condition of humidity in the
air, and the primers generated sulphuric acid from the action of
the moist air on the sulphur and the potassium chlorate.
At this time a typical rim fire primer used in the .22 caliber
ammunition had the following composition as obtained by chemical
analysis in the Frankford Arsenal Laboratory. This was from the
United States Cartridge Company's "N.R.A." .22 caliber Outdoor
type cartridge, loaded with Lesmok powder.
Potassium Chlorate .... 41-43%
Antimony Sulphide .... ..9.53%
Copper Sulphocyanide ....4.70%
Ground Glass ........... 44-23%
The average weight of the priming in this .22 rim fire cartridge
was found to be 0.237 grain.
[356]
The American ammunition companies and the shooters too, were
apparently quite unconscious of any trouble from the priming com-
position until Dr. Huff published his findings in 1922. But
evidently the Germans had been at least 20 years ahead of us in
this field, for we find, as quoted by Major J. C. Gray of the
Ordnance Technical Staff in his article "Lighting the Fire" in
the American Rifleman for January 1928, that the publication
Zeitschrift fur das Gesampte Schiess-und Sprengstoffwesen for
March 15, 1914 states "Attempts to manufacture a priming
composition which does not cause the barrel to rust were begun
about 1901." Also, "The first rust-free primers were made in 1901
by the Rheinische-Westphalische Sprengstoff A. G. in 1901."
The German composition contained barium nitrate in the place of
potassium chlorate, together with some picric acid to strengthen
the mixture. The formula was as follows:
Fulminate of Mercury .....39%
Barium Nitrate ...........41
Antimony Sulphide .........9
Picric Acid ...............5
Ground Glass ..............6
It also developed that the Swiss Army had been using a
Noncorrosive primer since about 1911. This was based oil the
formula of a Swiss inventor named Zlelyler. The Swiss formula was
Fulminate of Mercury....40%
Barium Nitrate .........25
Antimony Sulphide.......25
Barium Carbonate.........6
Ground Glass ............4
The barium nitrate replaced the potassium chlorate, and the
barium carbonate was added, probably to neutralize the acid
products of combustion.
As far as I can see, there was no the Germans or the Swiss to
keep these things secret. Apparently the information was there
available trouble to read the foreign scientific significance of
what he saw there.
As a matter of fact, it was quite well known in shooting
circles in this country just before World War I that the Germans
had been using since 191 1 what was known as the Rostfrei
(rustfree) primer in at least one brand Of .22. caliber rim fire
ammunition. However, not much notice was taken of it. There was
very little foreign .22 caliber ammunition sold here, and thou(Th
a few own editors spoke of it, no one seemed to grasp the fact
that a whole new idea in ammunition had been laid at their feet
until Dr. Huff's report woke them up.
[357]
This German "R" primer had the following composition:
Fulminate of Mercury.......55%
Antimony Sulphide .........11
Barium Peroxide ...........27
T.N.T ......................7
It will be noted that there was no chlorate in this picture.
But when attention had first been called to it, there were rumors
that this primer was very erosive on gun bores on account of a
gritty barium carbonate formed during the combustion. That is one
reason why no more attention was paid to it in this country.
As soon as the Huff report was released, both Frankford Arsenal
and all the commercial companies woke up and started working on
the development of a non-corrosive primer. The first to place a
satisfactory mixture on the market was Remington, with their
Kleanbore primer. This was the development of Mr. J. E. Burns, a
chemist on the Remington staff. This came on the market in 1927,
and was followed in short order by other brands and makes.
All of these early primers, like the German ones, contained
fulminate of mercury, and on account of this ingredient, they all
suffered from the serious disadvantage of short life in storage.
Frankford Arsenal made a chemical analysis of these early non-
corrosive priming mixtures, with the following result:
Remington
Fulminate of Mercury ..............44.40%
Barium Nitrate ....................30.54
Lead Sulphocyanide .................4.20
Ground Glass.......................20.66
Binder (gum, etc.)..................0.20
Western
Fulminate of Mercury ..............40.79%
Barium Nitrate ....................22.23
Lead Sulphocyanide .................8.22
Ground Glass.......................28.43
Binder (gum, etc.)..................0.33
Winchester Staynless
Fulminate of Mercury ..............41.06%
Barium Nitrate ....................26.03
Lead Sulphocyanide .................5.18
Ground Glass.......................26.66
Binder (gum, etc.)..................0.58
Peters Rustless
Fulminate of Mercury ..............38.68%
Barium Nitrate .....................9.95
Ground Glass.......................28.43
Undetermined lead compound.........25.91
Binder (gum, etc.)..................0.56
These mixtures all tended to become insensitive and to suffer
from hangfires and misfires after storage for a year or two,
especially in a warm damp place. This was due to the action of
the fulminate of mercury which they contained. This lead to the
eventual discovery of various substitutes for the fulminate, and
all current mixtures are of the non-mercuric type.
Two typical non-mercuric primer compounds are those patented by
Edmund Ritter Von Herz and Hans Rarhburg, and acquired from them
by the Remington Arms Company shortly after the introduction of
the first Kleanbore. These formulae follow:
Composition No. 1
Guanyl Nitro-amino-guanyl-tetracene ..0.5 to 15.%
Lead Tri-nitro-resorcinate ............20. to 45.
Barium Nitrate ........................30. to 50
Antimony Sulphide or other fuel or
both, as e.g. Calcium Silicide ........10. to 30.
[358]
Composition No. 2
Guanyl Nitro-Amino-guanyl-tetracene.........0.5 to 2.
Lead Tri-nitro-resorcinate ................35. to 40.
Barium Nitrate ............................35. to 42.
Lead Peroxide ..............................7. to 12.
Antimony Sulphide ..........................0. to 5.
Calcium Silicide ...........................0. to 12.
Glass.......................................0. to 3.
Naturally the Ordnance Department was quite anxious to use a
non-corrosive primer in the service ammunition, and a number of
experiments to this end were made at Frankford Arsenal, but the
mixtures tried either did not give good ignition or else failed
to stand storage, or developed some other disability.
As the Swiss were using a noncorrosive primer in their regular
service ammunition, there seemed no good reason why we should not
do the same, especially as we knew what the Swiss composition
was. A study of samples of Swiss ammunition showed that the
weight of the primer pellet they used was considerably greater
than that of ours. We were using about all the mixture we could
get into the primer cup, and it was impossible to (yet in as much
composition as the Swiss used. This was because we used a primer
known as the Boxer type, having the anvil of the primer in the
primer cup itself, while the European nations used the Berdan
primer, in which there was no separate anvil; instead, there was
a raised section of metal in the bottom of the primer pocket of
the case which acted as an anvil.
The primer with the Berdan anvil, integral with the cartridge
case, allowed more room for an increased char e of mixture.
Moreover, it gave an opportunity for more direct ignition of the
powder, for the flash holes lead directly from the primer to the
powder, while in our Boxer type, the flash hole is located under
the center of the anvil. The flash has to go through some cut out
places in the edge of the anvil, then come back to the center and
go down the flash hole to reach the powder. In other words, it
more or less has to go around a corner to get through the flash
hole.
It should be noted that the Berdan Primer is an American inven-
tion, which no doubt would be used in America today if it had not
been for the fact that it is difficult to extract these primers
for reloading. They have two small flash holes, located on each
side of the primer pocket, instead of one large one in the
center. These holes are much too small to permit the passage of a
de-capping punch. The Boxer type primer with its self-contained
anvil was developed to permit easy de-capping for handloading
purposes.
Now the Europeans all use the Berdan primer, and moreover, the
Western Cartridge Company, who made 8mm Lebel cartridges for the
French during World War 1, made them with the Berdan primer, and
liked it. A du Pont representative for a long period kept more
[359] or less pressure on Frankford to adopt the Berdan primer,
in the interest of better ignition.
All this resulted in the development of a non-corrosive primer
by Frankford Arsenal, which seemed to give superb results in
primer components of the Berdan type. This primer composition
differed from the Swiss type in that it contained no fulminate of
mercury. Fulminate has the very bad habit of deteriorating
rapidly in moist humid climates, such as we encounter in our
tropical possessions, though in a climate such as the mountainous
one of Switzerland, it is quite satisfactory. It has another
disadvantage from our viewpoint, and that is the fact that
cartridge cases fired with mercuric primers become brittle.
Thus we could not do the obvious thing and copy the Swiss
mixture, but we did succeed in getting another mixture which
seemed even better. The first large scale trial of this was at
the National Matches of 1930, where Frankford Arsenal submitted
for test by the Ammunition Board a lot of National Match
Ammunition loaded with non-corrosive primers in Berdan primers.
This lot of ammunition won the test with one of the best
accuracy records ever achieved, and was duly issued for use in
the National Matches. Shortly after the matches started, there
was a spell of abnormally hot weather at Camp Perry, and it was
found the ammunition was giving evidences of high pressures. The
Ordnance Department thereupon withdrew this lot and substituted
another which had the regular primer, and announced that
experiments with Berdan primers would end forthwith.
I greatly regret that I was absent in Europe at that time with
our International Rifle Team, and therefore did not see at first
hand. Just What happened. It would seem that with every other
nation in the world having used Berdan primers in every kind of
climate for many years, the trouble might have been due to some
other factor. The ammunition we used in Europe that year had the
Berdan primer, and the team won the World Championship and almost
everything else in sight. This, however, was low velocity
ammunition, especially designed for best accuracy at 300 meters.
The Berdan Primer fiasco spelled the end of the non-corrosive
primer in service ammunition for many years. Development pro-
ceeded feverishly, and all the commercial ammunition was changed
over to the non-corrosive type, but the rigid Government
specifications as to storage, hangfires, etc., could not be met
by these mixtures. Frankford Arsenal developed some promising
compositions, but none gave satisfactory results in the hangfire
test, which was important in those days of synchronized aircraft
guns.
This was the situation when mobilization occurred in 1940. Im-
mense orders for .30 Cal. A12 ammunition were placed with the
various cartridge companies, and there was quite a bit of talk
about non- corrosive [360] primers by some of the companies, who
thought they were prepared to make them; but when the time came
to sign on the dotted line, none of them were willing except the
Canadian firm of Dominion Industries, Ltd., who in 1945 made
100,000,000 rounds of cal. .30 M2 ammunition having a
non-corrosive primer.
At the present writing, March, 1947, we are changing over
slowly to a non-corrosive primer in the service cartridge, and a
portion of this year's manufacture will have this type of primer.
The change-over will proceed as fast as it can be done without
too much of a disruption of production until all ammunition is
being made that way, which should be in the very near future.
--------------------------
[485] Recent Developments
Non-Corrosive Primers
As will be seen by reference to pages 347, 348 and 349, the
cause of the destructive corrosion which had for so many years
plagued gun users was finally traced to salt deposited in the gun
barrel by the decomposition of potassium chlorate which had been
used for many years as one of the principal ingredients of most
small arms primers. Knowing that some European nations, notably
the Swiss and the Germans had developed primers which were said
to be completely noncorrosive, I made a trip to various European
cartridge factories and arsenals in 1927, and obtained the
formula for the non-corrosive primers that were then in use
there. On my return to Frankford Arsenal, where I was then
stationed, the laboratory at that place embarked on an extensive
development program to produce a non-chlorate primer that would
be satisfactory for military use.
The first move was to duplicate the very successful Swiss
primer. It was found, however, that their primer mixture was less
concentrated and more bulky than ours, and so required more room
in the primer cup than was available in primer cups of the
standard American design, containing a separate anvil.
Consequently, to use the Swiss mixture, or any of several
variations of it that we developed, it became necessary [496] to
go to the type of primer used in Europe, which has an integral
anvil formed from the brass of the cartridge case at the bottom
of the primer pocket, and having two (or three) small flash holes
at the sides instead of one large one in the center, as is the
practice with the American primer.
For the National Matches of 1930, an experimental lot of non-
corrosive primers was made up using the Berdan type of primer
construction. However, that year the weather was excessively hot
at Camp Perry (the site of the National Rifle Matches), and
trouble was experienced with high pressures. As a result, the
Ordnance Office in Washington ordered that experiments with the
Berdan type of primer be discontinued.
During further experiments with the Swiss type of primer
mixture (see P- 356), it was found that it has a serious defect,
which is the fact that it uses fulminate of mercury as an
important ingredient. Fulminate of mercury, has two disadvantages
for use in primers. One is tile fact that a cartridge case in
which a fulminate of mercury primer has been fired is adversely
affected by the mercury released, so that tile case is no longer
suitable for reloading or any other reuse as a cartridge. An even
more serious disadvantage of mercury fulminate is the fact that
primers containing it are likely to deteriorate with storage.
Some of the commercial ammunition companies when they first
produced noncorrosive 22 caliber rim fire ammunition used mercury
fulminate as a primer ingredient, and found that after several
years on the shelf the ammunition gave bad hang-fires or
mis-fires, or even became completely dead and insensitive.
No doubt the Swiss did not have that trouble because in their
comparatively cool and dry climate such deterioration was greatly
retarded, or perhaps never occurred at all.
Meanwhile, the Remington Arms Co., originators of the non-
corrosive priming known as Kleanbore, had obtained the services
of James E. Burns, a chemist who had about the mid-1920's
demonstrated to them an experimental non-corrosive primer that
lie had made in which he had found it possible to omit potassium
chlorate. An important ingredient in ]us primer was lead
tri-nitro-resorcinate, usually called lead styphnate.
It had also been found that instead of the troublesome
fulminate of mercury, it was possible to use a substance called
tetracene, a product of the reaction of amino-guanidine nitrate
with sodium nitrite. This is otherwise known as
guanyl-nitros-amino-guanyltetracne or guanyl-
diazo-guanyltetracene, and an application for a U. S. patent on
its use in primers had already been filed by Hans Rathbürg, of
Furth, Germany, in 1923.
The Remington Arms Co. purchased this patent, No. 1,586,380,
dated May 25, 1926, and also acquired patent No. 1,859,225, for
which application had been filed Jan. 5, 1929 by Edmund Von Herz
of [487] Cologne-Dellburg, Germany, and which covered the use of
lead styphnate in primers.
Enjoying the protection afforded them by these patents, Remington
produced the highly successful Kleanbore non-corrosive primers.
Other companies made up their own non-corrosive primer formulas,
and some of the early, ones came to grief through deterioration
of the fulminate of mercury used in them; but before long, all
companies had discarded fulminate, and all had come up with
highly satisfactory non-corrosive small arms primers.
Finally, the patents mentioned above ran out, and other
companies besides Remington used the styphnate primer mixtures
with various proportions of the different ingredients.
Meanwhile, Frankford Arsenal was continuing its development
work on the problem of developing a non-corrosive primer which
would meet the stringent requirements imposed by its use in
service ammunition. A highly important requirement is the ability
of the ammunition to undergo long periods of storage in tropical
climates without deterioration. Another requirement, and a most
important one from the viewpoint of the ammunition maker, is ease
of production and the absence of any tricky idiosyncrasies making
it necessary to demand extraordinary purity of the ingredients.
A burnt child dreads the fire, and Frankford Arsenal was still
smarting from the experience of World War I (see P- 355), when,
due to a certain combination of circumstances connected with rush
of war production plus the difficulty of obtaining completely
pure ingredients, the whole primer production of the Arsenal went
bad just when it was needed most. The F.A. No. 70 primer mixture,
adopted at that time, was so free from any such trouble, and so
utterly reliable, that the Ordnance Department, and Frankford
Arsenal in particular, dreaded any change in the primer
composition, and leaned over backward in making doubly sure that
any new mixture adopted would be satisfactory from every
viewpoint. Thus the adoption of a noncorrosive primer seemed to
lag during a seemingly interminable search for perfection.
About 1940 when the carbine had been adopted by the Army and
large contracts were about to be awarded to commercial ammunition
makers for caliber 30 carbine ammunition, Col. E. H. Harrison,
Ord. Dept., USA, was the officer in charge of preparing the
specifications on which this ammunition was to be purchased. He
decided that the piston arrangement of the carbine was too
vulnerable and too difficult to get at to risk primer corrosion,
so he simply put in the specifications the requirement that the
primer must be "non-corrosive" without specifying any primer
mixture or type.
By this time, every one of the companies which was awarded a
contract for the manufacture of carbine ammunition had developed
a satisfactory non-corrosive primer composition of its own, using
a lead [488] styphnate mixture, the basic patents above mentioned
having by then expired.
Thus each company making carbine ammunition used its regular
primer, and as far as anyone in the service could determine, they
all worked equally well. Through this far-sighted action on the
part of Col. Harrison it came about that all carbine ammunition
has non-corrosive primers.
Meanwhile Frankford Arsenal's search for a perfect
non-corrosive primer for other service ammunition had been
progressing, and they had come up with a non-corrosive primer
mixture consisting of barium nitrate and red phosphorus, and
started its manufacture.
While this was in many ways an excellent primer, it had two
disadvantages. The red phosphorus suitable for use in this primer
had to be of such extraordinary purity that it turned out to be a
problem to obtain it of the right quality and in sufficient
quantity; and moreover if the phosphorus came in contact with the
metal parts, an undesirable reaction occurred, so that the metal
components had to be protected against such contact with the
phosphorus.
This primer mixture was used for a time (about 1949) with
succcess [sic]; but it was finally decided to adopt a lead
styphnate primer mixture for all service small arms primers, and
such a non-corrosive small arms primer based on lead styphnate
was standardized by Ordnance Committee action in August, 1949.
The new mixture was put into production as soon as possible, and
as a consequence all small arms ammunition made since January,
195o, has primers of the non-corrosive type. Incidentally, they
are also non-fulminate, as fulminate of mercury was dropped as a
primer constituent about 1899 on account of the fact that at that
time it was the practice to return fired cartridge cases to
Frankford Arsenal to be reloaded, and the use of fulminate of
mercury in primers ruined the brass of the cartridge cases for
this purpose.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
Last chlorate corrosive US mil ammo (I believe) was 1950's Frankfort Arsenal. - Issue was made more serious as the primer cups may have eventually
lost some of the materiel within the (brass) casing. |
I suffer from the belief that this is the most common small arms primer.
Primer Rifle Pistol 'Sinoxid' aka PA-100
Stig Peterson
Explosion Products and Temperatures of the "Sinoxid" Percussion Primer
Föredrag vid Pyroteknikdagen 1971
Stockholm de 10 maj 1971
And I believe the Norma Reloading Handbook
Lead Trinitroresorcinate 38%
Tetracene 2
Barium Nitrate 39
Lead Dioxide 5
Calcium Silicide 11
Antimony Sulphide 5
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Wizard, as long as we are on this subject and our discussion does deal with oxidizers - I think your damn smart not to put that old stuff through your
baby. - You know where I'm going here: IF the corrosive primers have their chemicals dislodged and have empties (even a minute amount in to your
cartridge; you may have a serious over pressure situation which is causing you to have to use extreme measures to dislodge the shell.
But let's face it, 1982 is pretty darn new for a corrosive primer to be used in production (especially non-war time production where there would be no
problems w/ strategic materials.
However, laying the Devil's advocate here, it's within the realm of possibility that the head-stamp is not representative of the year but rather
something like the facility....(I'm reaching here; I know).
If there IS a way to disassemble them; it may be an idea to try and check it out. There ARE high pressure AIRCRAFT oriented .30's and .50's that are
super hot. They had been made fora variety of aircraft weapons that had extremely beefed up guns and operated in super cold conditions.
That book DOES sound like a serious find.
I know that because of the diameter, it's tough to disassemble the 50BMG, but realistically; you could use the metal components from it certainly and
it could put some $ in your pocket to keep a few if you find anyone who is a "50 collector" and wants some examples of that cartridge.
From what I remember, Lead Stephnate, Tetracene, Barium Nitrate, Lead Dioxide is a real standard. Interesting stuff.....
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
But let's face it, 1982 is pretty darn new for a corrosive primer to be used in production (especially non-war time production where there would be no
problems w/ strategic materials.
However, laying the Devil's advocate here, it's within the realm of possibility that the head-stamp is not representative of the year but rather
something like the facility....(I'm reaching here; I know).
If there IS a way to disassemble them; it may be an idea to try and check it out. There ARE high pressure AIRCRAFT oriented .30's and .50's that are
super hot. They had been made fora variety of aircraft weapons that had extremely beefed up guns and operated in super cold conditions.
|
Sportsmans Guide.com
http://shop.sportsmansguide.com/net/cb/cb.aspx?a=640209
Mighty .50 BMG 702 - gr. FMJBT Ammo.
1970s-production, 702-gr. steel core Full Metal Jacket
Boattail bullet, "FNB" headstamp, corrosive.
Fabrique Nationale Herstal [FNB], Herstal, Belgium.
Muzzle Velocity: 2,850 F.P.S. Muzzle Energy: 12,660 ft.-lbs.
I could have bought mine here - I don't remember. I bought a
bunch when prices started going up. If I get down to this lot I'll
pull the bullets and reduce the powder load. Popping 50 BMG live
primers... not me!
Someone was selling 50 BMG ammo stated to be
used with a heavy MG, definitely not in a Barrett. I do not
remember who it was. May have been the SM's Guide.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
It MAY just be some bizarre high altitude, designed for freezing temp rounds; designed for a very specific aircraft weapon. Bullets and brass are
still worth something. Dump the powder / bullet. You could just nullify the primer by a drop of oil or WD-40 and it shouldn't ignite from a
de-capper.
However, it MAY have triple base smokeless in it (nitroguanidine + NC / NG) which may be worth keeping. TBSP is occasionally used in 20-30mm cannon
rounds; it wouldn't surprise me if that's the powder you have there.
Seems everyone buys stuff from SG.... :-)
[Edited on 17-8-2010 by quicksilver]
|
|
|
| Pages:
1
2
3 |
|