Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
GUDN (Guarnylureadinitramide)
"GUDN is a dinitramide with excellent thermal stability, low water solubility and no hygroscopicity.
This substance has been proven to be an excellent fuel in gas-generating compositions where there are high requirements on thermal stability at high
temperatures and a high gas yield.
GUDN (FOX-12) can also be used in propellants for artillery.
It is a high explosive with calculated performance between TNT and RDX. Thanks to its extremely low sensitivity, GUDN could be a main component in
insensitive warhead fillings such as general purpose bombs, artillery, tank and mortar ammunition."
But where to get Guarnylurea to do the nitration?
I tried fusing sodium pyrosulfate with urea, and believe I have obtained Guarnylurea. The pyrosulfate pulls out water, dehydrating the 2 urea
molecules, rather than pulling out NH3 to make sulfamic acid. Sulfamic acid is known to fuse with urea to make guanidine sulfate for example.
Na2S2O7 + 2NH2CONH2 --> Na2SO4 + NH2C(NH2+)NHCONH3+
(SO4)-2
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
what nitration?
"The FOX-12 used for this work was synthesized using the following procedure: guanylurea sulfate hydrate (3.2 g, 0.01 mol) was dissolved in water (15
ml) and the pH was adjusted to 5–7 by dripping diluted sulfuric acid into the water. Thereby a clear solution was obtained. Ammonium dinitramide
(2.5 g, 0.02 mol) was dissolved in water (3 ml). The two solutions were combined and a precipitate was formed. The precipitate was collected and
washed with cold water and after drying guanylurea-dinitramide (3.4 g, 0.016 mol, 81%) was obtained as fine white crystals. Elemental analysis of the
product agrees well with calculated values: carbon 11.6% (11.5%), nitrogen 47.0% (46.9%) and hydrogen 3.4% (3.4%). Values within parenthesis are
calculated mass percentages for FOX-12. Identification of the substance was done by FT-IR, FT-Raman and X-ray analysis (see below). The crystal
density was measured using powder X-ray diffraction and found to be ρ=1.7545(4) g/cm3. A manuscript with the full single-crystal X-ray
diffraction investigation and ab initio quantum mechanical calculations is under preparation [12]. The heat of formation (ΔH°f) for FOX-12 was
calculated from a measured heat of combustion. The apparatus used for the experimental determination was an adiabatic bomb calorimeter of the IKA C
4000 type. The calibration of the calorimeter was done by combustion of certified benzoic acid in oxygen atmosphere at a pressure of 3 MPa. The heat
of formation was determined to be −355 kJ/mol."
Guanyl urea is available from dicyandiamide. This is available from calcium cyanamide.
http://www.google.com/url?sa=t&source=web&cd=7&v...
I have posted a DIY calcium cyanamide procedure long ago.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
This was my understanding also.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
N-GuanylUrea Dinitramide ( Fox 12 )
http://www.intdetsymp.org/detsymp2006/downloadmanuscript.asp...
append .pdf to open and view
GuanylUrea Dinitramide - Patent US6291711
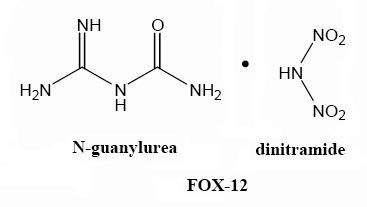
Newly investigated perhaps , not really all that new , at least in concept.
The insensitive nature , apart from being a salt , derives from the poor
oxygen balance and a base that is not very energetic to start with. It
offers no particular advantage over more readily made perchlorate salts.
If one is going to the trouble of making Dinitramide , insensitivity aside ,
a better product for the effort is Trinitromethane , which improves the
oxygen balance and number of gas mols produced , 10.5 instead of 9.
- with Trinitromethane :
2 { H2N.C(=NH).NHC(=O)NH2 • HC(NO2)3 } => 7 CO + 7 H2O + 7 N2
- with Dinitramide :
2 { H2N.C(=NH).NHC(=O)NH2 • HN(NO2)2 } => 4 CO + 6 H2O + 7 N2 + H2
- with Perchloric acid
2 { H2N.C(=NH).NHC(=O)NH2 • HClO4 } => 4 CO + 4 H2O + 2 HCl + 4 N2 + H2
- GuanylUrea Perchlorate - Patent US1298793
PATR - 2700 , D - 1217 ( Dicyandiamidine & Derivatives )
- Guanidine Perchlorate ( more sensitive )
PATR - 2700 , G - 152
http://www.sciencemadness.org/talk/viewthread.php?tid=1081&a...
- Biguanide Diperchlorate Patent US4340755
http://www.sciencemadness.org/talk/viewthread.php?tid=364&am...
If the performance is accurately reported this is comparable to Ethylenediamine Diperchlorate
http://www.sciencemadness.org/talk/viewthread.php?tid=13174
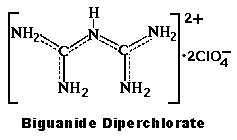
Salt adducts of organic bases with oxyacids collectively have not been
systematically and exhaustively investigated, at least not in any open
public domain literature. Available oxyacids are a small handful but possible
bases are limitless. Choosing one which is in itself energetic provides an
easy boost in performance. The reason there is much interest in high
nitrogen heterocycle bases Triazoles , Tetrazoles , Pentazoles.
The range of complexed salt compounds is little explored
bis-Triaminoguanidinium-bis-5,5-azotetrazolate ( sensitive , a primary )
http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=Get...
append .pdf to open and view
Compounds of common bases are all known
Ammonia , Hydrazine , Methyl and Dimethyl Hydrazine , Urea , Guanidine ,
Methylamine , Dimethylamine , Trimethylamine , Diaminoethane , Aromatics
and so on and so on.
N,N-Dimethyl-2,4,6-Trinitroaniline is more basic than Aniline and shows
there is no clear division between oxyacid and oxy base. This coupled with
Trinitromethane yields 17 mols of gas per compound unit.
9 CO + 3 H2O + 3.5 N2 + 1.5 H2
Acids and Bases review ( observation made in last page )
http://cms.ifm.liu.se/edu/chemistry/kurser/prep-org-kemi-1/a...
Obtained from Picryl choride and Dimethylamine.
Recrystallized from glacial acetic acid. Melting point 141 ºC
P. van Romburgh , Rec. trav. chim., 2, 105 ( 1883 )
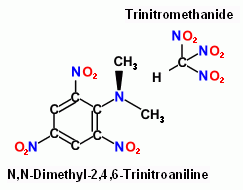
Oxyacids most commonly are Nitric or Perchloric , all other groups are poorly documented
Oxyacids which are themselves explosive also boost performance ,
in no particular order :
Nitramine and Dinitramine , Methylnitramine and Ethylenedinitramine ,
Nitrourea and Dinitrourea , Nitromethane and Trinitromethane ,
Trinitrophenol.
Low Sensitivity Energetic Materials
http://edoc.ub.uni-muenchen.de/8495/1/Welch_Jan.pdf
.
[Edited on 22-6-2010 by franklyn]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Actually, the "dinitramide", in this case, refers to the two nitramine groups, not a dinitramide anion. This is an interesting case in which the
chemical name leads to some ambiguity. While a dinitramide salt could certainly exist, it would probably not be as insensitive as the energetic
compound described.
O2NNHC(NH)NHC(O)NHNO2
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: | | But where to get Guarnylurea to do the nitration? I tried fusing sodium pyrosulfate with urea, and believe I have obtained Guarnylurea. The
pyrosulfate pulls out water, dehydrating the 2 urea molecules, rather than pulling out NH3 to make sulfamic acid. Sulfamic acid is known to fuse with
urea to make guanidine sulfate for example. Na2S2O7 + 2NH2CONH2 --> Na2SO4 + NH2C(NH2+)NHCONH3+ (SO4)-2 |
That is interesting.. Do you think there is a similar simple way to synthesize biguanide? That would be nice, I really like the looks of biguanide
diperchlorate.. The only ways I've seen to make it look very low yielding and require dicyandiamide.. Yes I realize dicyanamide can be made from home
made calcium cyanamide, but with that many steps involved I doubt it would be practical to scale up much (or at least less practical than PETN which
it could replace).
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I like the way you think. The biguanide diperchlorate would indeed be a good energetic salt. Treating Guarnylurea with anyhydrous NH3 might form the
biguanide, since I know that acetylamidine CH3C(NH)NH2 can be prepared in a similar way from acetic acid. Or it might just turn the Guarnylurea into
two guanidines. Perhaps fusing sulfamic acid with guarnylurea would work.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That book gives no reference for the claim of N,N-dimethyl-2,4,6-trinitroaniline being more basic than aniline. All literature reports on the
measurements of its pKa indicate that it is many million times less basic than aniline. Different references give different pKa's but it is always
about -5 or thereabout. In Journal of Physical Organic Chemistry, 13, 372-381 the authors made a table with pKa's supported by references and they list the pKa's of aniline
(4.58), p-nitroaniline (1.11), 2,4-dinitroaniline (-4.54), 2,4,6-trinitroaniline (-9.41), N,N-dimethylaniline (5.15), N,N-dimethyl-p-nitroaniline
(0.60), N,N-dimethyl-2,4,6-trinitroaniline (-4.98) and pKa's of numerous other anilines and amines. Though the effect of the N,N-dimethylation is
dramatical (pKa changes from -9.41 to -4.98) and does indeed increase the basicity of N,N-dimethyl-2,4,6-trinitroaniline by nearly 30000 times when
compared to its nonmethylated variant, the compound still remains practically unprotonable by anything but the most extremely acidic acids and
practically unprotonable in aqueous media.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Quote: Originally posted by Anders Hoveland  | Actually, the "dinitramide", in this case, refers to the two nitramine groups, not a dinitramide anion. This is an interesting case in which the
chemical name leads to some ambiguity. While a dinitramide salt could certainly exist, it would probably not be as insensitive as the energetic
compound described.
O2NNHC(NH)NHC(O)NHNO2 |
I think you will want to check the crystal structure of GUDN/FOX12, or the synthesis I posted above, or the structure franklyn posted...
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I guess to avoid confusion you would have to call it:
alfa-, omega-dinitro guanylurea
or
1,5-N,N dinitro guanylurea
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ Nicodem
O O P S , thanks for pointing that out.
Still ,
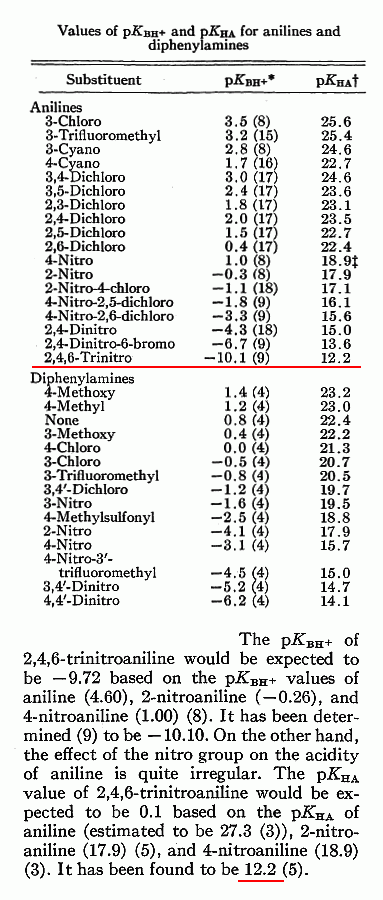
I understand that , the smaller the integer pKa value is , the stronger relative acidity.
In general pKa of ( 7 ) or above is regarded as somewhat basic. Aryl amines are less basic
than ammonia with a pKa of ( 9.24 ). Aniline I see given as ( 4.63 ) N,N-Dimethylaniline as ( 5.15 )
Here _ http://www.zirchrom.com/organic.htm
2,4,6 -Trinitroaniline is given here as ( 12.2 ) center of the chart on page 3
reproduced and attached below with the relevant excerpt from the text here _
A Comparison of the Acidity and Basicity of Aromatic Amines
http://pubs.nrc-cnrc.gc.ca/journals.old/cjc/cjc45/v67-156.pd...
The statement I referred to above on the last page here _
http://cms.ifm.liu.se/edu/chemistry/kurser/prep-org-kemi-1/a...
" 2,4,6-Trinitro-N,N-Dimethylaniline is much more stronger base than
N,N-Dimethylaniline or 2,4,6-Trinitroaniline "
If this is true , it must have a pKa which is greater than ( 12.2 ) definitely basic.
Trinitromethane and also Dinitraminic acid ( elsewhere discussed in this thread )
have negative integer values comparable to mineral acids such as nitric and sulfuric.
The acidity of Trinitromethane surely compensates for any weakness of basic attribute.
The formation of a 2,4,6 -Trinitroaniline salt adduct is also not a product seen from
it's production with mixed sulfuric and nitric acids so this data does not resolve
the issue.
Of course all this does not even account for molar concentration and nonaqueous
solvent effects which can be considerable, as observed in the lower attached image
excerpted from page 108 The Chemistry of Anilines Part 1 By Zvi Rappoport
Takes a while to get it from Google books here => http://tinyurl.com/22nczbg
- Caution :
" 2,4-dinitroaniline (- 4.54 ), 2,4,6-trinitroaniline (- 9.41 ) " not being compounds that
dissolve lime on contact , the figures quoted are particularly suspect , likely a misprint
or misidentified. Trinitromethane's pKa is ( - 5 ) and is more acidic than Sulfuric acid ,
any value more negative would be some flourine containing super acid.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Anders Hoveland  | the chemical name leads to some ambiguity. Actually, the "dinitramide", in this case,
refers to the two nitramine groups, not a dinitramide anion. O2NNHC(NH)NHC(O)NHNO2 |
@ Anders Hoveland
In what case ? This is not a substituted urea , which are covalent molecules.
You confuse covalent bonded Nitramine with ionic Dinitramide. There is no ambiguity
- Dinitramidic acid anion forms ionic salts , the suffix alone ( - i d e ) should tell you
that. ( In Organic nomenclature used for anions formed by lending one or more hydrogen
protons off the parent hydride: e.g. , Methanide , Nitramide , Hydrazinide ).
You have heard of neutralization and double decomposition ? Very useful techniques
when applied as in this case to proton base salts , no " nitration " involved at all.
and on another note =>
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
The explosive I originally described , in the first post of this topic, is possibly technically named incorrectly. However, the double nitramine is
still what was meant, not the dinitramide salt. You brought up the dinitramide salt, which is a different compound.
Why would they have formed a dinitramide salt with guarnyl urea, a compound that has two basic sites? The double dinitramide would have an excess
oxygen balance in the decomposition products, almost certainly not what would be found in an explosive that was meant to be insensitive.
If someone wanted to make a perchlorate salt, they might consider oxalamidine, which is likely easier to make than "bi-guanidine". Oxalamidine can be
made from Ammonium oxalate and anhydrous NH3. Its structure is NH2C(NH)C(NH)NH2, with a carbon-carbon bond.
Guanylurea can be nitrated to a double nitramine similar to guanidine nitration to nitroguanidine. Guanylurea sulfate can be the precursor in the
nitration, as con. H2SO4 is used in the nitration anyway.
[Edited on 24-6-2010 by Anders Hoveland]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Hmm I like the possibilities with oxalic acid based precoursers. Although I don't think you could make oxalamidine by the action of ammonia on an
oxalate. From what I've read it will only go to oxamide. But that could be interesting too, the dioxime of oxamide is basic according to literature..
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
NH3 on acetic acid is used in a synthesis described by "Megalomania" in the now defunct "Explosives and Weapons Forum" to make acetamidine, so I see
no reason why NH3 could not be used to make oxalamidine. Or perhaps NH2C(NH)CONH2.
By the way anhydrous N2H4 can react with guanidine to displace the ammonia, making (NH2NH)2CNNH2, this is possible because the trinitrate of
(NH2NH)2CNNH2 is less soluble in ethyl alcohol than guanidine nitrate. So NH2NHC2O2NH2 could likely also be made by precipitating it out with
phosphate. This is pure speculation of course.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Quote: Originally posted by Anders Hoveland  | The explosive I originally described , in the first post of this topic, is possibly technically named incorrectly. However, the double nitramine is
still what was meant, not the dinitramide salt. You brought up the dinitramide salt, which is a different compound.
Why would they have formed a dinitramide salt with guarnyl urea, a compound that has two basic sites? The double dinitramide would have an excess
oxygen balance in the decomposition products, almost certainly not what would be found in an explosive that was meant to be insensitive.
|
The copy/ paste you have in the first post is from here
http://www.eurenco.com/en/high_explosives/new_energetic.html
This makes FOX12 aka GUDN.
Searching these terms on scifinder gives only the ionic dinitramide salt.
Searching for the double nitramine covalent material gives no results.
Tell me, how do you know it forms a double nitramine, and that concentrated sulfuric can be used in the nitration?
"Why would they have formed a dinitramide salt with guarnyl urea, a compound that has two basic sites? "
Because half of energetics research is making as many salts as possible and seeing what has desirable properties. Same as drug screening.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by franklyn  | I understand that , the smaller the integer pKa value is , the stronger relative acidity.
In general pKa of ( 7 ) or above is regarded as somewhat basic. Aryl amines are less basic
than ammonia with a pKa of ( 9.24 ). Aniline I see given as ( 4.63 ) N,N-Dimethylaniline as ( 5.15 )
Here _ http://www.zirchrom.com/organic.htm
2,4,6 -Trinitroaniline is given here as ( 12.2 ) center of the chart on page 3
reproduced and attached below with the relevant excerpt from the text here _
A Comparison of the Acidity and Basicity of Aromatic Amines
http://pubs.nrc-cnrc.gc.ca/journals.old/cjc/cjc45/v67-156.pd... |
The value 12.2 is for the conjugate base, that is the acidity of 2,4.6-trinitroaniline. That is why the index "HA" which stands for Hydrogen-Acid.
This pKa value is about the ease of deprotonation.
The pKa = -10.1 is for basicity, thus having the index "BH" (Base-Hydrogen). This value gives the acidity of the conjugated acid (the acidity of
2,4,6-trinitroanilinium ion).
In this specific case you can estimate that NaOH (pKa(BH) of H2O is ~15) can efficiently and nearly quantitatively deprotonate 2,4.6-trinitroaniline
(equilibrium constant is about 10^3). Yet it is impossible to protonate 2,4,6-trinitroaniline in aqueous media (pKa(HA) of H2O is -1.7), except in
insignificant traces, because the equilibrium constant is above 10^8.
I hope this makes sense to you. The confusion is due to the habit of chemists of giving the basicity values as pKa's of the conjugated acids
(because there is no general scale for basicity). So when a functional group is at the same time protonable and deprotonable then it has two pKa
values, pK(HA) and pKa(BH). For example, methanol has aqueous pKa(HA) of about -2 and pKa(BH) of about 15. This gives some ambiguousness, because it
is often up to the reader to figure out if the pKa given is pKa(BH) or pKa(HA).
Edit: Could someone please tell the guy who posts referencelles nonsense that it is spelled GUANYL and not GUARNYL! It gives me shivers every time I
read that "guarnyl" thing. It is almost as annoying as the lack of references.
[Edited on 24/6/2010 by Nicodem]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Anders Hoveland  | NH3 on acetic acid is used in a synthesis described by "Megalomania" in the now defunct "Explosives and Weapons Forum" to make acetamidine, so I see
no reason why NH3 could not be used to make oxalamidine. Or perhaps NH2C(NH)CONH2.
By the way anhydrous N2H4 can react with guanidine to displace the ammonia, making (NH2NH)2CNNH2, this is possible because the trinitrate of
(NH2NH)2CNNH2 is less soluble in ethyl alcohol than guanidine nitrate. So NH2NHC2O2NH2 could likely also be made by precipitating it out with
phosphate. This is pure speculation of course. |
Things are not always logical in chemistry especially if you compare apples with pears and peaches! Each molecule has its specificities and sometimes
you can make some generalisation as a molecule enter a like-like familly...but before generalisation was done, it was tested empirically by
experiments proving or infirming the hypothesis-This is true for organic but also for mineral chemistry!
You are certainly not stupid, but sometimes you may look like by comparing HNO3 with HClO4 or by making shortcuts in your theories that look unclear
because of the lack of references...or too much ideas running from your brain to your fingers while writting...I don't know.
Typical example is the following:
"By the way anhydrous N2H4 can react with guanidine to displace the ammonia, making (NH2NH)2CNNH2, this is possible because the trinitrate of
(NH2NH)2CNNH2 is less soluble in ethyl alcohol than guanidine nitrate."
-It reacts with it and subsitute one, two and finally 3 amino groups by hydrazino ones; the reason has nothing to do with the solubility in ethanol of
the nitrate salts...it has to do with limits equilibriums forms of the guanidine and with the fact, despites hydrazine is less basic than ammonia,
hydrazine is less volatile than ammonia...so by the Le Chatellier's rules if one of the products leaves the system being more volatile; this will
drive the equilibrium in the sense of the products:
(NH2)2C=NH + H2N-NH2 (l) <=> (NH2)3C-NH-NH2
(NH2)3C-NH-NH2 <-=> (NH2)2C=N-NH2 + NH3(g)
(NH2)2C=N-NH2 <=> (NH2-NH)(NH2)C=NH
(NH2-NH)(NH2)C=NH + H2N-NH2 (l) <=> (NH2)2C(NH-NH2)2
(NH2)2C(NH-NH2)2 <-=> (NH2-NH)(NH2)C=N-NH2 + NH3(g)
(NH2-NH)(NH2)C=N-NH2 <=> (NH2-NH)2C=NH
(NH2-NH)2C=NH + H2N-NH2 (l) <=> (NH2)C(-NH-NH2)3
(NH2)C(-NH-NH2)3 <-=> (NH2-NH)2C=N-NH2 + NH3(g)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I found a Swedish defense paper clearly describing GUDN Fox-12 as the dinitramide salt. However, a google image search revealed a tiny picture and I
was just able to make out the dinitramine of guanylurea; it indicated that it came from Science Madness forum; however when I clicked on it, it came
up with an error, so I do not know where it was posted. It does not appear in this thread. The little summary under the picture described "cold
preparation", presumably because of a nitration. I will do some more searching. I would think the nitramine would be even more stable than the
dinitramide salt.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I am of the opinion that that dintramine of guanylurea would be more powerful and stable than the dintramide salt.
It has 1N and 3H atoms less than the dinitramide salt, so this is like taking away an ammonia molecule to make it more powerful. Further, ammonium
dintramide is known to be much more sensitive than the perchlorate. NitroGuanidine, the nitramine of guanine is extremely inert, it is formed by a
cold nitration of guanidine, and is easier to carry out than making an an organic nitrate ester, although it still requires a H2SO4 concentration
above 98%. Guanylurea can be similarly nitrated to the dinitramine.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Anders Hoveland  | Actually, the "dinitramide", in this case, refers to the two nitramine groups,
not a dinitramide anion. This is an interesting case in which the chemical name
leads to some ambiguity. O2NNHC(NH)NHC(O)NHNO2 |
Quote: Originally posted by Anders Hoveland  | The explosive I originally described , in the first post of this topic, is possibly
technically named incorrectly. However, the double nitramine is still what was meant, not
the dinitramide salt. You brought up the dinitramide salt, which is a different compound. |
Misnaming and subsequent mis-characterization of the compound first described
highlights a neglected area of investigation overlooked and poorly documented.
Structural iterations of Urea , Guanidine , and similarly Methylenediamine , namely
Biuret , Biguanide and Ethylenediamine all readily form adducts with oxo acids.
N-Nitro derivatives of these amine bases compared to the simpler Dinitrourea ,
Dintroguanidine and Methylenedinitramine , have enhanced stability. Although
Methylenedinitramine decomposes Ethylenedinitramine is stable. Although Dinitrourea
decomposes Dinitrobiuret is stable. Two different accounts of this later compound
differ in their assessment however. Earlier research is dismissive of it's utility. See
section 12 on document page 12 , pdf pg 16 , section 34 on document page 24 ,
pdf pg 28 , and property tables after document page 29 , pdf page 33 _ here
Compounds of High Nitrogen Content in Propellant Compositions
http://handle.dtic.mil/100.2/AD595292 redirects to:
http://www.dtic.mil/cgi-bin/GetTRDoc?AD=AD595292&Locatio...
More recent investigation recommends its consideration and application.
Attachment: Dinitrobiuret & its Salts .pdf (604kB)
This file has been downloaded 2065 times = = Attachment: Biuret from Urea.pdf (406kB)
This file has been downloaded 1905 times
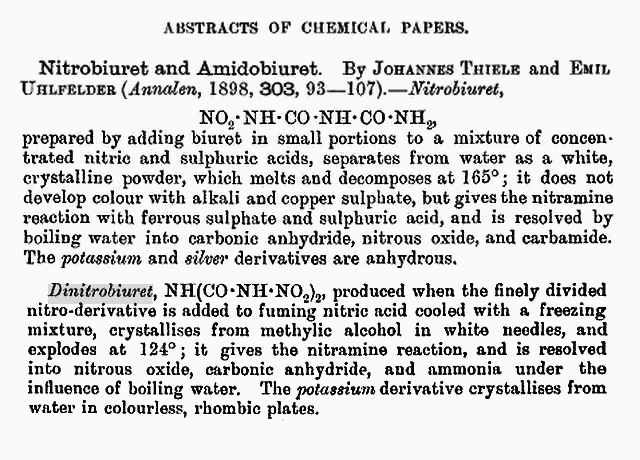
Recently isolated Dinitroguanidine and it's derivative Dinitrobiguanide show acidic
character and also form salts as do the other Nitramine compounds cited above.
See article page 509 , pdf pg 9 _ here
Synthesis and Some Properties of 1,2-Dinitroguanidine http://ifile.it/hik1spe
or here _ http://www.sciencemadness.org/talk/files.php?pid=187259&...
Attachment: Biguanide.pdf (99kB)
This file has been downloaded 2431 times
Guanylurea is a composite part Urea and part Guanidine no different than any of the
other variations of this theme convertible just the same into Dinitroguanylurea.
As an amine base it forms adducts as described below.
Low Energy Monopropellants Based on the Guanylurea Cation
http://peer.ccsd.cnrs.fr/docs/00/51/83/05/PDF/PEER_stage2_10...
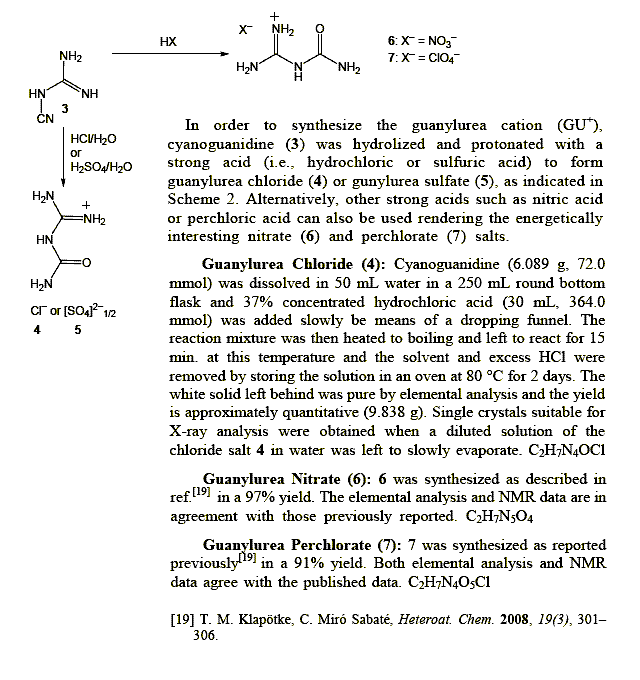
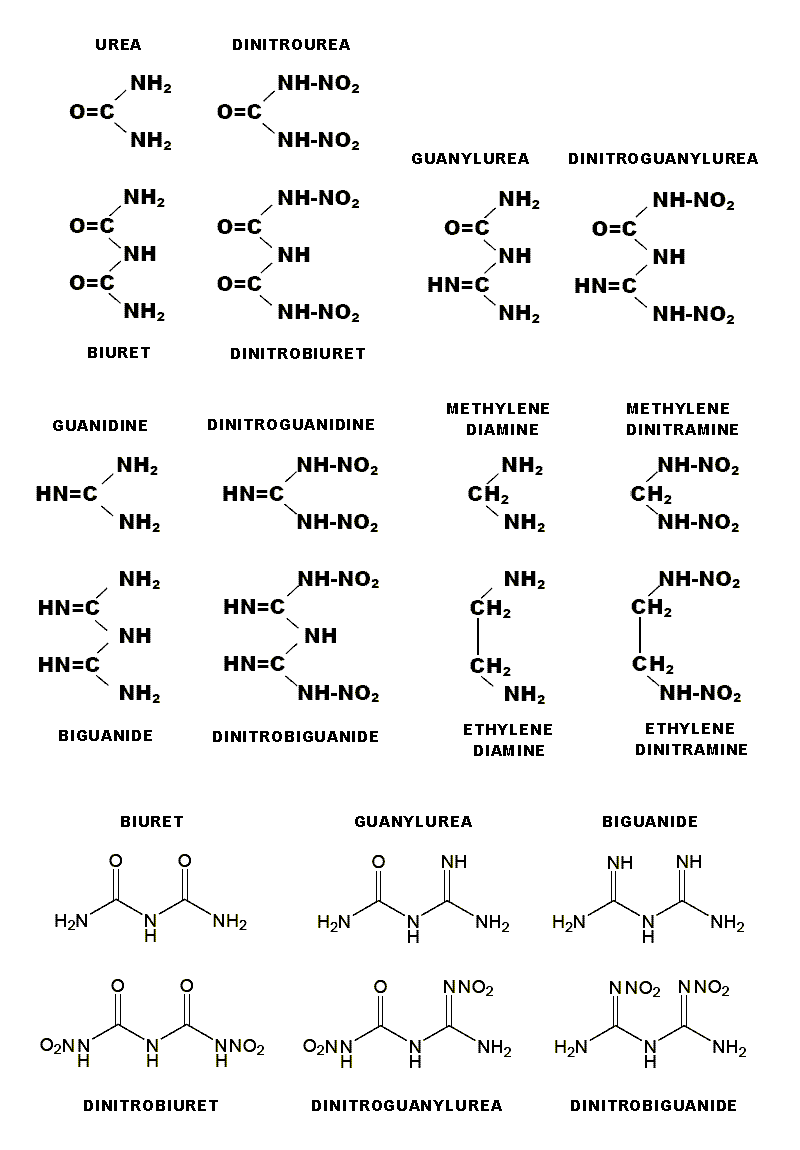
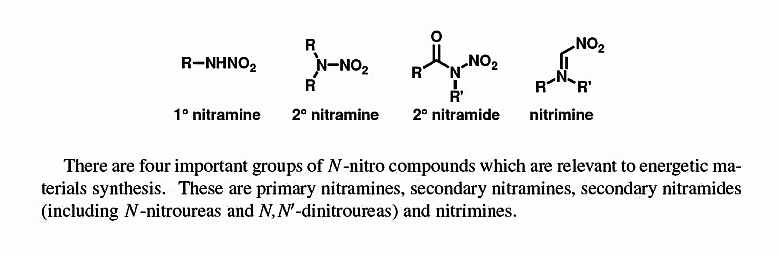
Tautomeric character can be expressed either as a Nitramine or else as
the Nitrimine form. In a related thread expounded on Nitroguanidine here _
http://www.sciencemadness.org/talk/viewthread.php?tid=9443#p...
.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Guanylurea, also called dicyandiamidine and dicyanodiamidine is monobasic, by the way structure and formula is given according to US1298793 and other
sources. Biguanide appears to also be monobasic, so it is curious that biguanide would form a diperchlorate, and this would appear possibly to be an
acid of crystallization or possibly an acid salt.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
The prospect of condensing Dinitrobiuret with formaldehyde is intriguing just
from the oxygen balance which is not that bad, ( Dinitrobiuret has + 4.1%
oxygen balance ) the monomer essentially adds a single carbon thus reducing
the oxygen balance by -12.4 % , http://en.wikipedia.org/wiki/Oxygen_balance
which compares well to PETN at -10.1 %.
simplified representation of the chain condensation
2 NH(CO.NH.NO2)2 + 2 CH2O=> 2 (CO.N(-).NO2)NH(CO.N(CH2)NO2) + 2 H2O
2 (CO.N(-).NO2)NH(CO.N(CH2)NO2) => 3 CO2 + 3 CO + 3 H2O + 5 N2
Comparison with nitrocellulose indicates what properties might be expected.
Nitrocellulose is a nitrate polymer unlike other discrete molecular organic
nitrates which are more sensitive to shock. A nitramine polymer will have
some similarity to the fibrous nitroguanidine. Note that it is ~ -31 % oxygen.
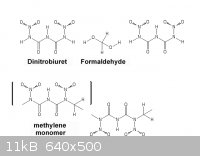
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I think the product would more likely resemble di-keto RDX, but with one less nitro group.
If this is correct, the chemistry of that N-H bond would closely resemble that in cyanuric acid. Likely it could be chlorinated to something like a
hybrid between RDX and TCCA. Then possibly react with dimethylamine to form a hydrazine derivitive. This should be high yield, because there would be
no hydrogen atoms on the final product, so it should not be vulnerable to destructive oxidation by additional unreacted still chlorinated molecules.
Then perhaps an energetic salt could be formed?
(NO2)2-[C3H2O2N3]-N(-CH3)2 *HNO3
(This last part of the post is pure speculation)
[Edited on 21-12-2011 by AndersHoveland]
|
|
|