Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
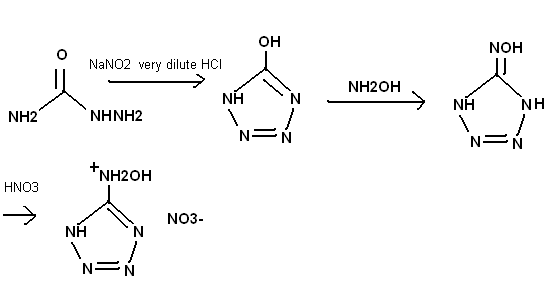
hydroxylamino-tetrazole (easy)
Also, guanidine is known to react in anhydrous N2H4 to form
(NH2NH)2CNNH2. The nitrate salt of this can be separated out from ethyl alcohol since it is much less soluble than guanidine nitrate. Urea might react
with chloramine to make NH2CONHNH2
The synthesis below is rather vague, but it does offer an interesting easy route to new energetic salts.
If anyone wants to attempt it, I would suggest reviewing a detailed synthesis of making tetrazole or tetrazine explosives.
Alternatively isocyanic acid and N2H4 should react to make NH2CONHNH2. HOCN can be made by heating urea to decomposition, first driving off NH3,
then decomposing the trimer of HOCN. The HOCN vapor must be quenched and cooled fast, otherwise it will polymerize back into the trimer. Preferably it
should be directly collected into the cold hydrazine.
[Edited on 20-6-2010 by Anders Hoveland]
[Edited on 20-6-2010 by Anders Hoveland]
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
There is already quite an extensive thread on 5-Aminotetrazoles, and similar compounds. Engager made a very nice procedure for creating 5-ATZ from
urea and ammonium nitrate in the prepublication section.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
By the way the addition of HNO3 in the last step must be very dilute, otherwise it will oxidize the hydroxylamine group. Dilute HClO4 could also be
used. This is a HYDROXYLaminotetrazole, and so should be a better oxidizer than plain aminotetrazole. A salt with hydroxylaminotetrazole cations and
the nitrotetrazolate anions, described by the Russian guy in the prepublication section, would be an interesting energetic material.
CN5OH4+ CN5O2-
|
|
|