| Pages:
1
2
3 |
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Quinone to make Energetic Compound
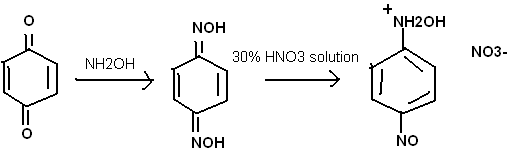
Alternatively, one could begin with nitro-quinone. The oxidation with nitric acid is tricky. You must use just the right concentration, and and not
use too much. Phenol would be a good solvent. The yield is low, as the nitric acid attacks the hydroxylamine group.
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
Reading this, I feel as though I just woke up in a lecture I didn't intend to be in.
Have you done this? What happened? Pictures?
What is this WIZardry?
/exit stage left.
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | The oxidation with nitric acid is tricky. You must use just the right concentration, and and not use too much. |
Ooh, trying even harder to make it sound like you've actually tried this. Frankly at this point I'm hoping you get banned. You haven't responded to
any of the requests to document your speculation with references. And your main response to criticism that you are engaging in idle flights of fantasy
seems to be to be deliberately vague as to whether what you're posting is experimental or hypothetical. It wouldn't be so bad if so much of your spew
weren't related to energetic materials; sooner or later I'm sure you'll post some brain fart that happens to be a perfect recipe for disaster, and I
don't really like to see the forums littered with such land mines.
|
|
|
Justin
Hazard to Self
 
Posts: 84
Registered: 6-5-2010
Member Is Offline
Mood: No Mood
|
|
Sadly I feel the same, your info would be much more useful if there were some references to the synthesis of these theoretical compunds, or atleast
some experimental data done by you.
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Nitration of benzoquinones or hydroquinone might be interesting idea I had recently, anyone documented it!?
I assume that o and p isomers would have interestingly different properties.
Question for someone who is more in organics;
Is there even remote possibility to put -ONO2 group in place of -OH in hydoquinone just like in
alcohols!?
That sounds like a long shot,but I am pretty sore you could put both,-NO2 and -ONO2 on molecules
like safrole or any phenyl-alkene or phenyl-alkene with alk(a/e)ne bigger than 3 C.
Phenyl compound with -ONO2 would be interesting!
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
No, you cannot put a NO3 in place of the OH, and still have another OH at the opposite end. This arrangement automatically would revert to HNO3 and
quinone, sine the H on the OH would ionize and the electron on the O would move over to the NO3.
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
No need for -OH on the opposite end,can you supstitute them both with -ONO2!?
In other case,can you put -NO2 on the phenyle and -ONO2 on the propene/propane group !?
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
That is a difficult question. I am not completely sure. I highly doubt it could be done by a direct nitration. Perhaps para dibromobenzene and AgNO3
in benzene.
Even then the para dinitrate of benzene would hydrolyze and self oxidize in water.
[Edited on 23-6-2010 by Anders Hoveland]
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
I just asked is it possible,I don't expect it ot be stable or useful.
Is there any documented phenyl compund with both -NO2 and -ONO2 groups!?
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Actually, benzene 1,4-dinitrate likely shares a resonance state, meaning it would have an equilibrium with 2 NO2 and quinone. The dinitrate might be
stable under dry ice, but probably quinone is far too stable to revert back in any significant ammount.
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Why is it 1,4-dinitrate so unstable while 1,3,5-trinitrobenzene is not,by "stable" I mean it can be handled at room temperature.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Maybe Ar-O-NO2 might exist but then it will be as a transcient species because it will be a kind of mixte anhydride of two acids, like acetyl nitrate
(CH3-CO-O-NO2); but, unlike this later, it will display a reactive counterpart into the aromatic moety that will unvariably lead to ortho- or
para-nitrophenol what are more stable thermodynamically.
Ar-O-NO2 <--==> Ar-O(-) + NO2(+)
H-C6H4-O(-) + NO2(+) <--==> O2N-C6H4-O(-) + H(+) <==> O2N-C6H4-OH
Same kind of phenomenon is observed with aniline...that yield sometimes a nitramine prior to rearangement into nitroanilin!
[Edited on 23-6-2010 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
OK,lets make it simple...
You can put -NO2 on the phenyle/(A) without problem.(TNT,TNP...)
You can put -ONO2 on alchohols/(B)without problem. (NG,ETN,EGDN...)
Can you put B on the A and have both -NO2 and -ONO2 groups on the same molecules!?
For example...Safrole(or anything alike without metilen dioxy ring if it tempers with reaction)!?
Longer the A the better!
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
To answer the three questions:
1,4 dinitrate would be unstable because of resonance. The benzene has delocalized bonding electrons, so the NO2's could break off without "breaking"
any other bonds or creating radicals, because the two O atoms still on the benzene become doubly bonded onto the carbons.
1,4 dinitro benzene would be stable, because nothing can break off without creating radicals (with unpaired electrons).
"H-C6H4-O(-) + NO2(+) <--==> O2N-C6H4-O(-) + H(+) <==> O2N-C6H4-OH"
You forgot two of the equilibrium reactions;
(-)ON(+)=C6H4=O H(+)
O(-)
O2N--C6H5=O
One of these last two would probably be the most stable.
However, I am fairly sure that PhONO2 does not ionize. In contact with a concentrated strong acid, it would ionize in equilibrium, and the NO2+ would
likely nitrate an adjacent position next to the now ---OH2+ group on the benzene. Thus you would get 2-nitrophenol.
The last post I did not understand. Yes, you can put a nitrate and nitro on the same compound. Now if you mean, can you make 1-nito, 4-nitrate
benzene, the answer is: I am uncertain but my guess would be yes. This compound might share a resonance with (-)(-)O2N(+)=C6H4=O NO2(+),
but I doubt it would ionize without a strong acid.
[Edited on 24-6-2010 by Anders Hoveland]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by mfilip62  | OK,lets make it simple...
You can put -NO2 on the phenyle/(A) without problem.(TNT,TNP...)
You can put -ONO2 on alchohols/(B)without problem. (NG,ETN,EGDN...)
Can you put B on the A and have both -NO2 and -ONO2 groups on the same molecules!?
For example...Safrole(or anything alike without metilen dioxy ring if it tempers with reaction)!?
Longer the A the better! |
Off course you can get both nitro and nitrate groups in an aromatic ring! Especially if you have the alcoholic moety appart from the aromatic ring...
Typical examples are:
-2,4,5 trinitrophenethyle nitrate: O2NO-CH2-CH2-C6H2(NO2)3
-1,3,5 trimethylol-2,4,6 trinitrobenzene trinitrate: C6(NO2)3(-CH2-ONO2)3
It is possible than a phenyl nitrate exists longer than as a transcient species if the aromatic ring can't react further with the NO2(+)...I imagine
it could be the case in pentamethylphenol.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by mfilip62  | Nitration of benzoquinones or hydroquinone might be interesting idea I had recently, anyone documented it!?
I assume that o and p isomers would have interestingly different properties.
.....
Phenyl compound with -ONO2 would be interesting!
|
I think you should check into the nitranilate tread.
I also have very good ideas about o- and p- nitroquinones.
You have a very powerfull and beautifull molecule that is hexamethylolbenzene hexanitrate (C6(-CH2-NO3)6) that proves you are right in writting what
you wrote.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Anders Hoveland  |
"H-C6H4-O(-) + NO2(+) <--==> O2N-C6H4-O(-) + H(+) <==> O2N-C6H4-OH"
You forgot two of the equilibrium reactions;
(-)ON(+)=C6H4=O H(+)
O(-)
O2N--C6H5=O
One of these last two would probably be the most stable.
However, I am fairly sure that PhONO2 does not ionize. In contact with a concentrated strong acid, it would ionize in equilibrium, and the NO2+ would
likely nitrate an adjacent position next to the now ---OH2+ group on the benzene. Thus you would get 2-nitrophenol.
The last post I did not understand. Yes, you can put a nitrate and nitro on the same compound. Now if you mean, can you make 1-nito, 4-nitrate
benzene, the answer is: I am uncertain but my guess would be yes. This compound might share a resonance with (-)(-)O2N(+)=C6H4=O NO2(+),
but I doubt it would ionize without a strong acid.
[Edited on 24-6-2010 by Anders Hoveland] |
Actually I didn't forgot, I simply did kept it simple...resonance forms and hybridations are often way to complex to write down here and doesn't say
much or add much to the text.
If following you Ar-O-NO2 doesn't ionise without a concentrated strong acid...how do you account for the fact acetyl nitrate is a strong NO2(+)
generator?It is usually used without external acids
Also a simple experiment I have done is to mix toluen and 69% HNO3 (what is, you must admit, far from being a concentrated HNO3 full of NO2+); I found
strange I was able to get after a very short while (minutes or hours) without agitation nor heating (ambiant temperature) an interfacial yellow colour
that turned day after day darker and darker (orange, brown,black with a green shade)...in fine after a few monthes I got orange to yellow cristalls
that filled all the toluene layer...this simple observation went in total contradiction with my belief (based on what I have learned at university or
in books) that concentrated HNO3 is needed to achieve aromatic nitration.
I believe what I got is paranitrotoluen, maybe with some orthonitrotoluene and nitrobenzoic acid...but I have to make some tests on it...I did take a
lot of pictures of the reactor and so I will try to post those down here later.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Thanks!
If you have the whole synthesis and some info on properties of hexamethylolbenzene hexanitrate (C6(-CH2-NO3)6)
please post it! (or PM me at least)
I am glad someone confirmed my idea and this -CH2-NO3 has sense,it "widens" the molecule and gives enough space
for aditional -ONO2 gruops.
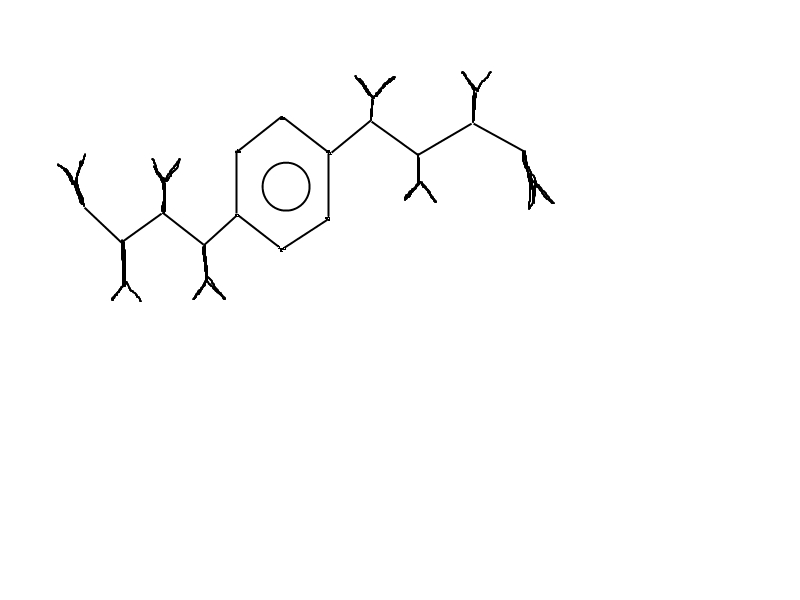
Anotherinteresting examle might be to put etilo,propilo,butilo or longer group(s) on phenyl (no need to max. number of six)
and than "grow" some nice -ONO2 groups on them like beries! 
I don't want to be smart-ass and say some organic molecules name that are proably wrong,so here is
very fast and rough sketch where "Y" might be -ONO2 groups.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
If acetic nitrate can indeed introduce a NO2 group onto phenol, then I would not think that phenyl nitrate would be stable. Does anyone know why
acetyl phenol would be able to nitrate toluene, but not itself? One would think it could react with acetic acid, generating nitroform or CO2. I could
not find much information about the compound, or whether it is even just a transient species. Nitric acid oxidizes acetic anhydride,(see the topic
about tetranitromethane) so why would acetyl nitrate be expected to exist?
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Define stable!?
Some people (like most of my professors) label every explosive as "unstable" molecule, even TNT !
In world of energetic materials, everything that can be handled without causing havoc is stable.
Molecules that tend to explode on tiniest provocation or they exist only under extreme circumstances
(P,T,light wavelength...) are unstable.
|
|
|
psychokinetic
National Hazard
   
Posts: 558
Registered: 30-8-2009
Location: Nouveau Sheepelande.
Member Is Offline
Mood: Constantly missing equilibrium
|
|
Indeed, stability is relative to what you want it for!
“If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found
the object of his search.
I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor.”
-Tesla
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by mfilip62  | Thanks!
If you have the whole synthesis and some info on properties of hexamethylolbenzene hexanitrate (C6(-CH2-NO3)6)
please post it! (or PM me at least)
I am glad someone confirmed my idea and this -CH2-NO3 has sense,it "widens" the molecule and gives enough space
for aditional -ONO2 gruops.
Anotherinteresting examle might be to put etilo,propilo,butilo or longer group(s) on phenyl (no need to max. number of six)
and than "grow" some nice -ONO2 groups on them like beries! 
I don't want to be smart-ass and say some organic molecules name that are proably wrong,so here is
very fast and rough sketch where "Y" might be -ONO2 groups.
|
The synthesis of hexamethylolbenzene hexanitrate (C6(-CH2-NO3)6) is not easy...I have a reference of this compound somewhere in books it is listed as
relatively unsensitive (impact sensitivity less sensitive than PETN and as brisant has HMX) for a polynitrate ester...probably because there are no
adjacent (vicinal) nitrate groups....PETN has nitrate groups on carbons separated by a carbon atom and are thus not vicinal...this accounts for its
"unsensitivity" as compared to the other nitrate esters of the H-(CHONO2)n-H familly (with n = 1,2,3,4,5,6)....same occurs with CH3-CH2-ONO2 (ethyl
nitrate) and CH3-CH(ONO2)-CH2-CH2ONO2 or O2N-CH2-CH2-CH2-CH2-ONO2 (butandiol dinitrate).
The synthesis goes from hexamethylbenzene, to hexakis-chloromethyl-benzene via a radicalar halogenation....then I suppose they hydrolise it to the
alcool with a base and finally nitrate it with conc HNO3.
I would shortcut the hydrolyse and the nitration by methatesis with AgNO3..because chloromethylaromatics are benzilic halogen and thus very easily
subsitued!
White unsoluble AgCl is easily collected and recycled!
AgCl -sunlight-> Ag + 1/2 Cl2 (g)
Ag + HNO3 --> AgONO2 + NOx
Ar-CH2-Cl + AgONO2 --> Ar-CH2-ONO2 + AgCl (s)
Thus from
C6(CH2Cl)6 + 6 AgONO2 ---> C6(CH2ONO2)6 + 6 AgCl
The molecule you designed O2NOCH2-(CH(ONO2)-)3-C6H4-(CH(ONO2)-)3-CH2ONO2 will be dense but less powerfull than, and as sensitive to shock as, mannitol
hexanitrate (hexanhexol hexanitrate).
[Edited on 24-6-2010 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Damn, you are really got at this! 
I just give example how phenyl might have both -ONO2 and -NO2 groups.
I didn't draw them,but -NO2 might be added also,
Just put those alkane groups on benzene so they make enough space to add -ONO2
on them and -NO2 on phenyl rign.
I have another idea how to remove vicinal distortion and have 12 -ONO2 groups.
Of course this might be extremely difficult to do,but rotation of propane groups may be just enough
to generate enough space for -ONO2 groups.
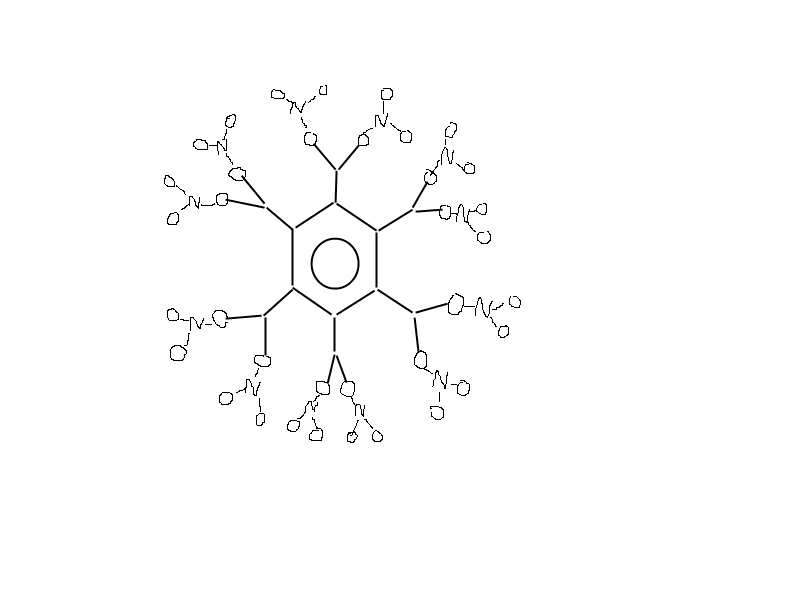
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
All this seems pointless to me. Why not distribute those nitates over all 12 of the carbons to make it more stable? Even if two nitrate groups on the
same carbon would not oxidize a C--H bond, your compound would be very unstable, violently decomposing in even the slightest trace of water, as the
dinitrate would hydrolyze to become 2HNO3 and O=CH---R, as the concentrated HNO3 would readily oxidize everything.
Something like O2NOCH2CH(NO3)CH(NO3)...etc
[Edited on 25-6-2010 by Anders Hoveland]
|
|
|
mfilip62
pierced by a crossbow under a bridge while eating Billy goats
  
Posts: 140
Registered: 25-8-2006
Member Is Offline
Mood: I like turtles!
|
|
Sorry,I made a little mistake in drawning,but I tough exactly that.
Those "Y" groups are actually 1,3-Propanediol conected to the phenyl with middle C atom,while two other (end) C atoms
that had -OH on them are nitrated,times 6.
|
|
|
| Pages:
1
2
3 |