Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
1,2,2 trinitro propane
Nitrogen dioxide can be prepared far more easily than concentrated nitric acid. Nitrogen dioxide reacts with excess acetone to create NitroAcetone
(and H2O and nitric oxide). This is because acetone is a ketone and there is a double bond between carbons in equilibrium. The double bond makes one
of the hydrogen atoms come off easier. For example, acetone reacts with bromine at room temperature. This reaction is somewhat slow, unless the pH is
lowered, but in this case that would ruin the reaction. First the NO2 has to stop being bubbled in. Then a 20% HCl solution is added. This causes the
the NitroAcetone to hydrolyze with water to hydroxylamine chloride and pyruvic acid. For example, NH2OH is made commercially from nitromethane and
sulfuric acid, formic acid being the byproduct in that case.
Hydroxylamine will condense with more NitroAcetone to create an oxime. CH3C(NOH)CH2NO2. This then reacts with nitrogen dioxide to yield
CH3C(NO2)2CH2NO2. This is quite resistant to shock, and in fact more powerful than NG. Yes, more powerful. NG has more oxygen atoms but some of them
are already bonded to carbon, not all the oxygen available will get reduced, and anyway the formation of 3 moles of carbon monoxide produces more
energy than 2 moles of CO2, so an energetic compound should not always aim toward complete oxidation. Furthermore, two nitro groups on the same carbon
add extra energy.
NO2 can be prepared from a nitrate, a mildly concentrated strong acid (20% solution), and a metal, the nitric oxide escaping reacting with air.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Just how gullible do you think we are Anders?
http://www.sciencemadness.org/talk/viewthread.php?tid=4132#p...
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
No doubt many of the reactions used are rather esoteric and not commonly known of. And I would not blame anyone for thinking these reactions are too
bizarre to work. I do not know which particular reaction in the synthesis is in doubt.
1. Acetone does indeed react with nitrogen dioxide
Nitrogen dioxide on an alkene will form the nitroalkene. Of course the gas does not react (unless combusted) with an alkane such as heptane.
CH3C(O)CH3 has an equilibrium with CH2C(OH)CH3. The latter begins to dominate as the pH is lowered, that is basic textbook stuff.
2. Nitromethane reacts with HCl solution. CH3NO2 + H2O + HCl --> HCOOH + NH3(OH)Cl.
By the way hexamine reacts with a concentrated HCl solution in a similar way. Alternatively NH4Cl will react with 2 CH2O, and a water molecule, to
form CH3NH3Cl, and HCOOH.
3. NH2OH does indeed condense with a ketone to form an oxime =NOH. Ammonia, by contrast, only transiently forms an imine =NH so there is only a
small condensation product in equilibrium. The industrial production of hydrazine sometimes uses acetone to improve yield (on wikipedia).
4. I saw a while back on a forum (either this one or the Explosives and Weapons Froum, which is now bombshock forums) that an CH3C(NOH)CH3 reacts with
NO2 to make CH3C(NO2)2CH3. I drew the reaction mechanism out on paper and it seemed reasonable that the reaction could be expected to happen, despite
my initial surprise that two nitro groups would go on the same carbon.
5. I looked at the link provided above and, yes, a nitration on acetone cannot be performed with nitric acid. A nitro group IS INDEED initially added
to the acetone, but since HNO3 is so acidic, the hydroxylamine is immediately formed from the nitro group (see point #2) and hydroxylamine, a good
reducing agent, quickly gets oxidized. This is why the pH cannot be allowed to go too low when performing the nitration with NO2, otherwise NH2OH will
form and NO2 will oxidize it. NaNO2 buffer might help.
Finally, NG might seem to many people like an ideal explosve, but in fact there are many others more powerful that simple oxygen balance analysis
would not explain, for example DADNE, (NH2)2CC(NO2)2 is significantly stronger than NGlycerine.
[Edited on 16-6-2010 by Anders Hoveland]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Anders, your post gave the impression that 1,2,2 trinitropropane was a known and studied compound, rather than a purely hypothetical one.
But if what you say is correct and you can prepare it as described, by divulging it here, you've probably shot yourself in the foot as far patenting a
novel energetic material is concerned. . .
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: | | I saw a while back on a forum (either this one or the Explosives and Weapons Froum, which is now bombshock forums) that an CH3C(NOH)CH3 reacts with
NO2 to make CH3C(NO2)2CH3. I drew the reaction mechanism out on paper and it seemed reasonable that the reaction could be expected to happen, despite
my initial surprise that two nitro groups would go on the same carbon. |
I don't know if its quite that simple.. According to this ketoximes react with NO2 (N2O4) to form "pseudonitroles" which is the term for a 2-nitro-2-nitroso alkane.. So to get your 2,2-dinitro you
would have to oxidize the nitroso, which is also mentioned in the book..
Also I'm unconvinced that 1,2,2-trinitropropane would be particularly stable.. From what I remember some other polynitroalkanes can be quite unstable,
having that acidic hydrogen and all..
[Edited on 17-6-2010 by 497]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | Also I'm unconvinced that 1,2,2-trinitropropane would be particularly stable. |
One nitro group attached directly to an aliphatic carbon is likely to be destabilising - and two doubly so. . .
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
1,1,3,3- tetranitro cyclobutane (TNCB) has a higher det. velocity than HMX, and I remember reading that it was more impact resistant than TNAZ, which
is, if I remember correctly, more insensitive than RDX. I cannot find the website, however. Unfortuneately TNCB is extremely difficult to synthesize,
despite its ideal properties, TNAZ is only moderately difficult by comparison.
Of course DADNE is an example of a very stable compound with two nitro groups on the same carbon, but that is totally different because of the
resonance with the amine groups.
I have also designed a synthesis for several potentially new compounds, such as 1,1,4,4-tetranitro, 2,3,5,6-tetramino benzene; if anyone is interested
I can post it.
[Edited on 17-6-2010 by Anders Hoveland]
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
| Quote: | | 1,1,3,3- tetranitro cyclobutane (TNCB) has a higher det. velocity than HMX, and I remember reading that it was more impact resistant than TNAZ, which
is, if I remember correctly, more insensitive than RDX. |
Yes I think you're right. It is possible that it would be stable. From what I've read 2,2-dinitropropane is relatively stable and has been
manufactured on at least pilot plant scale as a diesel cetane rating improver.. So it must be at least reasonably stable, but that additional nitro
might have a negative effect on that. By the way 2,2-dinitropropane is a solid melting not too far above room temperature. I don't for sure, but I
would imagine an additional nitro group would raise its melting point..
Interestingly 2,2-dinitropropane is one of the only nitroalkanes that improves cetane ratings, which is because of the weaker C-N bonds on a
gem-dinitroalkane. So I could say with confidence that it would be less stable than nitroalkanes with only one nitro per carbon. Stable enough for
practical use? Hard to say..
If the yields involved in your proposed reaction scheme were good, I think it could potentially be viable. You might have problems with multiple steps
having low yields, especially the oxidation of the pseudonitrole seems to be inevitably low yielding. But all the materials may be available and cheap
enough that a low yield is acceptable.
PS
Another possible variation could be nitrosating the acetone instead of nitrating it. NaNO2 + HCl in DMSO will nitrosate methyl ketones.. The nitroso
at the 1 position would probably be oxidized along with the one at the 2 position, so the product should end up the same.. I don't know quite how it
would effect the other steps in the reaction other than requiring a different source of hydroxylamine. I suppose that may defeat the whole simplicity
of the process...
[Edited on 18-6-2010 by 497]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by Anders Hoveland  |
I have also designed a synthesis for several potentially new compounds, such as 1,1,4,4-tetranitro, 2,3,5,6-tetramino benzene; if anyone is interested
I can post it. |
You're certainly brimming with ideas Anders, and on close inspection they do look quite interesting.
So post away. . .
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
I have designed molecular structures, for instance. In addition, 1,2,4-tri(nitromethylene) pentazole has a surprising number of resonances in the
5-membered nitrogen ring, could be expected to be very powerful, and decently stable.
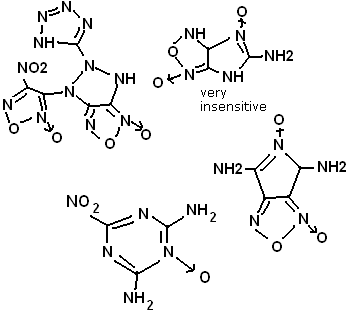
[Edited on 18-6-2010 by Anders Hoveland]
[Edited on 18-6-2010 by Anders Hoveland]
[Edited on 18-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Analine and Cl2O6 (which has a ClO2+ in it) might make the intermediate phenylNHClO2 (and an analine perchlorate). The compound (benzene 1-NHClO2,
2-NO) should have a resonance with what is in the picture, with an extra H+ of course, that could be neutralized by NH3)
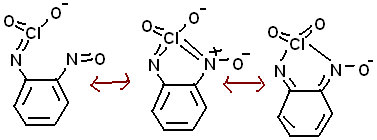
[Edited on 18-6-2010 by Anders Hoveland]
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
Also, this has many resonance states, so could be expected to be stable. Plus you could stick on 2 extra nitro groups (not shown)
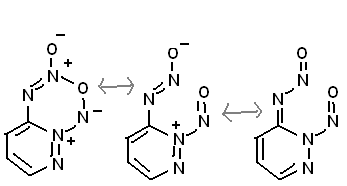
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
1,1-Dinitroethane
To a stirred solution of 2.66 g (66.5 mmoles) of sodium hydroxide in 15 ml of water at 20° was added 5.0 g (66.5 mmoles) of nitroethane. When all the
nitroethane dissolved, the solution was cooled to 5°-7° in an ice-water bath.
The sodium salt of nitroethane was prepared as above. To the stirred solution was added a solution of 20 g (288 mmoles) of sodium nitrite in 50 ml of
water, followed by a solution of 4.4 g (13.4 mmole) of potassium ferricyanide in 25 ml of water. Finally, 16.0 g of solid sodium persulfate (67
mmoles) was added all at once. The reaction temperature, moderated by an ice-water cooling bath, increased to 50°. The orange mixture was stirred at
25° for 1 hour and then cooled to 10°. Urea, 20 g (0.33 mole), was added, followed by 10 ml of glacial acetic acid. The mixture was extracted with
three 25 ml portions of ether and the combined extracts were washed with brine and dried. The crude product was distilled to give 4.2 g (52% yield) of
1,1-dinitroethane, b.p. 87°-89° (16 mm), identified by its nmr spectrum.
Patent 4910322
32.5 grams of ethyl bromide (0.3 moles) was poured into a stirred solution of 600ml dimethylformamide and 36 grams dry NaNO2 (0.52 mole) in a beaker
standing in a water bath keeping the solution at room temperature as the reaction is slightly exothermic. Always keep the solution out of direct
sunlight. The stirring was continued for six hours. After that, the reaction mixture was poured into a 2500 ml beaker or flask, containing 1500 ml
ice-water and 100 ml of petroleum ether. The petroleum ether layer was poured off and saved, and the aqueous phase was extracted four more times with
100 ml of petroleum ether each, whereafter the organic extracts were pooled, and in turn was washed with 4x75ml of water. The remaining organic phase
was dried over magnesium sulfate, filtered, and the petroleum ether was removed by distillation under reduced pressure on a water bath, which
temperature was allowed to slowly rise to about 65°C. The residue, consisting of crude nitroethane was distilled under ordinary pressure (preferably
with a small distillation column) to give 60% of product, boiling at 114-116°C.
32.5 grams of ethyl bromide (0.3 moles) was poured into a stirred solution of 600ml dimethylformamide and 36 grams dry NaNO2 (0.52 mole) in a beaker
standing in a water bath keeping the solution at room temperature as the reaction is slightly exothermic. Always keep the solution out of direct
sunlight. The stirring was continued for six hours. After that, the reaction mixture was poured into a 2500 ml beaker or flask, containing 1500 ml
ice-water and 100 ml of petroleum ether. The petroleum ether layer was poured off and saved, and the aqueous phase was extracted four more times with
100 ml of petroleum ether each, whereafter the organic extracts were pooled, and in turn was washed with 4x75ml of water. The remaining organic phase
was dried over magnesium sulfate, filtered, and the petroleum ether was removed by distillation under reduced pressure on a water bath, which
temperature was allowed to slowly rise to about 65°C. The residue, consisting of crude nitroethane was distilled under ordinary pressure (preferably
with a small distillation column) to give 60% of product, boiling at 114-116°C.
Nitro Ethane
32.5 grams of ethyl bromide (0.3 moles) was poured into a stirred solution of 600ml dimethylformamide and 36 grams dry NaNO2 (0.52 mole) in a beaker
standing in a water bath keeping the solution at room temperature as the reaction is slightly exothermic. Always keep the solution out of direct
sunlight. The stirring was continued for six hours. After that, the reaction mixture was poured into a 2500 ml beaker or flask, containing 1500 ml
ice-water and 100 ml of petroleum ether. The petroleum ether layer was poured off and saved, and the aqueous phase was extracted four more times with
100 ml of petroleum ether each, whereafter the organic extracts were pooled, and in turn was washed with 4x75ml of water. The remaining organic phase
was dried over magnesium sulfate, filtered, and the petroleum ether was removed by distillation under reduced pressure on a water bath, which
temperature was allowed to slowly rise to about 65°C. The residue, consisting of crude nitroethane was distilled under ordinary pressure (preferably
with a small distillation column) to give 60% of product, boiling at 114-116°C. Ethylene glycol also works as solvent, but the reaction proceeds
pretty sluggishly in this medium, allowing for side reactions, such as
CH3CH2-NO2 + CH3CH2-ONO -> CH3CH(NO)NO2 + EtOH. KNO2 can also be used instead of NaNO2. If NaNO2 is used in DMF, 30g of urea can also be added as
nitrite scavenger to minimize side reactions, as well as simultaneously increasing the solubility of the NaNO2 and thereby significantly speeding up
the reaction.
If the ethyl bromide is substituted with ethyl iodide, the required reaction time is decreased to only 2.5 h instead of 6 h. In case ethyl iodide is
employed, a slight change in the above procedure needs to be done. The pooled pet ether extracts should be washed with 2x75ml 10% sodium thiosulfate,
followed by 2 additions of 75ml water, instead of four of 75ml water as above, to remove trace leftover I2.
Alternate Nitro Ethane
1.5 mole sodium nitrite (103.5g) is intimately mixed with 1 mole of sodium ethyl sulfate (158g) and 0.0625 moles of K2CO3 (8.6g). The mixture is then
heated to 125-130°C, at which temperature the nitroethane distills over as formed. heating is discontinued when the distillation flow slackens
considerably, and the crude nitroethane is washed with an equal amount of water, dried over CaCl2, The nitroethane is then re-distilled, collecting
the fraction between 114-116°C. Yield 46%
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
1,1,1-trinitro ethane
An idea for making 1,1,1-trinitro ethane.
NO2 is known to react with an oxime (R2C=NOH) to make R2C(NO)(NO2). Possibly NO2 would react with
ethyl nitrolic acid CH3C(NOH)(NO2) to make
CH3C(NO)(NO2)2, which then might react with dilute H2O2 to give the final product of CH3C(NO2)3. This would be very powerful, but I am unsure how
stable it would be. It might require a booster charge to detonate or it might explode if thrown to the ground.
Also I found something on 1911 encyclopedia Britanica:
"The silver salt (silver nitroformate), obtained by shaking an ether solution of nitroform with freshly prepared, slightly moist silver oxide, reacts
with methyl iodide to form trinitroethane, a crystalline solid which melts at 56° C. Concentrated caustic potash decomposes the latter compound,
forming the potassium salt of dinitroethane,
CH3 C(N02)2K"
quoting another post,
"US patent 5043488: slow addition of nitric acid to acetone produces an unknown explosive", probably ethylnitrolic acid (1-Nitro-1-oximinoethane).
CH3C(NOH)NO2
"was prepared by mixing acetone with nitric acid (of 24% concentration) and a little fuming HNO3 and allowing the mixture to stand for 8 days at room
temperature. An ether extraction gave on evaporation some acetylmethylnitrolic acid
Beil 3,621 and
R. Behrend & H. Tryller, Ann283,221- 3( 1894)
"... right after the strong oxidation left behind was a clear, thick yellow oil of a pungent odor. According to Jahresbericht über Fortschritte der
Chemie (1902), Behrend and Tryller, p. 1075-77 this oil contained one-third to half acetylmethylnitrolic acid (CH3.CO.C(NOH).NO2) (cryst., mp. 62
deg., very decomposable) and other byproducts, including pyruvic and oxalic acids. They say in the same instance methyethyl ketone affords CH3COOH,
HCOOH, and considerable amounts of ethylnitrolic acid and dinitroethane"
There is a slight chance that using methyl ethyl ketone, which is easily obtained as a paint solvent, might also make, in very low yield, some
CH3CH2C(NOH)NO2, which could then be used to make 1,1,1-trinitropropane.
I also found something else interesting (from wikipedia)
"A small amount of hydrazine blended in nitromethane can increase the power output even further. With nitromethane, hydrazine forms an explosive salt
that is again a monopropellant. This unstable mixture poses a severe safety hazard."
Confirmation that Nitromethane can indeed acts as an acid to form salts: "sodium nitromethane, CH 2: NO(ONa) "
This salt would likely form a powerful combination with hydrazinium perchlorate. A hydroxylamine salt would also be likely to form HONH3(+)
H2C=N(O)--O(-).
It should be pointed out that CH3NO2 is more energetic than TNT, but since it is a small molecule, it suffers from low density, which diminishes its
potential power. A good route to design energetic compounds is to start with something very energetic, but with low density, and then figure out how
to make the molecule biger or pack the molecules closer together. This is far easier than starting with a good high density explosive and trying to
figure out what you can add to make it more powerful. The larger molecule nitro alkanes should be highly energetic, while being more insensitive than
is possible with nitramines.
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
No2 and fuel explosive patent
Here is a very interesting patent I ran across.. It seems like a very easy on-site binary explosive. Having the power in weight ratio to tnt.
http://osdir.com/patents/Explosive-charges/Nitrous-oxide-bas...
Any thoughts on needed pressures in a confined container to achieve the most power..? Why not create a pvc tank that is injected with the proper
weighed propane, then on site a nitrous oxide whip-it is injected into the tank. The explosive is said to be impact sensitive and can be initiated
with a spark.
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
This "patent" does not sound very useful other than for
making "bombs". It would be hard to use this mixture in
a bore hole or shaped charge and the author makes no
mention of VOD or anything to indicate that he actually tried it.
Propane is liquid below about 80psi and I think it is 800psi
for N2O so you will need a very strong container.
There are probably many mixtures which which would form
binaries and be easier to use (although not spark sensitive) perhaps conc. HNO3 and methanol?
|
|
|
Hoveland
Harmless

Posts: 20
Registered: 20-7-2010
Member Is Offline
Mood: No Mood
|
|
According to Jared Ledgard, in "The prepatory manual of explosives" (3rd edition),
1,1,1-trinitro, 2-hydroxy ethane can be prepared by refluxing trinitromethane (nitroform)
with trioxane (formaldehyde) at 60degC.
I also found a book if anyone has a strong interest in energetic materials and is willing to bear with some advanced organic chemistry, and much
complexity:
(Br)(NO2)2C-CH2OC(=O)CH3 reacts with 2KI to make:
CH3C(=O)OCH2C(NO2)2(-) K(+), KBr, and I2.
1,1,1-trinitroethane can undergo 2 reactions on treatment with base, depending on what the base is. First, and the least unusual, the base can react
with the molecule to form the 1,1- dinitroethane anion. The second reaction involves a base abstracting a hydrogen from 1,1,1-trinitroethane, followed
by elimination of nitrous acid, forming a reactive intermediate of 1,1-dinitro ethylene, which can further react with R3C(-) to form
R3C-CH2-C(NO2)2(-)
or it can react with R2NH to form
R2N-CH2-CH(NO2)2 where the R could be a methyl
Reaction of 1,1-dinitro ethylene with KOEt gives 2,2-dinitro ethyl ether in 80% yield.
K(NO2)2C-CH2-OC(=O)CH3 reacts with 1,1-dinitro ethylene to make
K(NO2)2C-CH2-C(NO2)2-CH2-OC(=O)CH3, which can then react with 70%H2SO4 to make
H(NO2)2C-CH2-C(NO2)2-CH2OC(=O)CH3, a potential useful precursor for insensitive energetic material.
CH2=C(NO2)2 also reacts with K(NO2)2C-CH2OH to make
K(NO2)2C-CH2C(NO2)2-CH2OH, when acidified, this leaves
(NO2)2CH-CH2C(NO2)2-CH2OH
Note: I would NOT suggest a nitration on this last compound, lowering the pH might be problemetic at the other end of the molecule, but on the other
hand, the molecule apparently survived 70%H2SO4 without hydrolysis,
so apparently the (NO2)2CH--R group is not as vulnerable as nitromethane is known to be.
“Organic chemistry of explosives” By Jai Prakash Agrawal
I was unable to cut and paste, so as a result I could only manually write down some of the things that stood out most.
[Edited on 24-7-2010 by Hoveland]
|
|
|
pjig
Hazard to Others
  
Posts: 169
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
You think that under low pressures that this intimate gas mix wouldn't go high order?
| Quote: | Propane is liquid below about 80psi and I think it is 800psi
for N2O so you will need a very strong container. |
Im assuming that under pressure you gain weight and density, due to the gases condensing to liquid form, meaning you just get more volume of
explosive in one area. If it were to be used in a long length of pipe capped ,dropped into a borehole and pressurized,it should go high order, and be
able to replace other charges.
Quote: Originally posted by gregxy  | This "patent" does not sound very useful other than for
making "bombs". It would be hard to use this mixture in
a bore hole or shaped charge and the author makes no
mention of VOD or anything to indicate that he actually tried it.
Propane is liquid below about 80psi and I think it is 800psi
for N2O so you will need a very strong container.
There are probably many mixtures which which would form
binaries and be easier to use (although not spark sensitive) perhaps conc. HNO3 and methanol? |
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
The mixture may go high order but the power is still going to be proportional
to the density. A solid or liquid has about 1000X the density of a gas at
atmospheric pressure. So to make an effective explosive requires a strong
heavy, metal container to get the pressure up to 800psi to keep the N2O liquid.
Also N2O is made by heating NH4NO3. From a cost & practicality basis using the
NH4NO3 and some fuel (like Al) would be a better choice.
If you really want a spark sensitive gas system you can generate H2 & O2 in
an electrolytic cell or better yet use acetylene and O2 from a welding set up.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
| Quote: |
2,2-Dinitropropane, a white crystalline (melting point, 51-52 °C), is generally considered too thermally unstable for use. Held at 75 °C for 48
hours in a closed container, it loses two-thirds of its weight.
"Explosive Effects and Applications", Jonas A. Zukas, William Walters, p155
|
Another idea might be to react bromoform with the sodium salt of nitromethane. (the sodium salt of nitromethane would need to be kept in pure alcohol
since it undergoes further reaction with water, bromoform can be made from a modification of the haloform reaction, considering that hypobromite is
not stable so must be made in reaction, and that bromoform much more readily hydrolyses with water than chloroform)
This might make 1,3,4-trinitroisobutane, CH(CH2NO2)3
There does not appear to be any information about it in the literature.
[Edited on 21-2-2012 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Here is a quote from researchers in St.Petersberg Russia;
"reaction of nitroethane salt with sodium nitrite . . . 1,1-dinitroethane formed in a high yield."
N.A. Petrova, Zhurnal Organicheskoi Khimii Volume 43, # 5, p. 653–656. (2007)
|
|
|
|