Devilinajolie
Harmless

Posts: 17
Registered: 16-3-2009
Member Is Offline
Mood: No Mood
|
|
MgSO4 decomposition
Hi everyone , i have a question about magnesium sulfate ( MgSO4 ) decomposition by heating please , when you heat ( what's the exact temperature
please ) MgSO4 after a period of time ( time please ) do you obtain SO2 or SO3 ?
Thanks again
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Asking to be spoon fed on something this easy ?
You really must learn how to do your own research.
The very first place to look _ http://en.wikipedia.org/wiki/MgSO4
Wikipedia claims 1124 ºC _
More authoritative sources :
CRC handbook states 1127 ºC for decomposition
According to the Mellor reference on Magnesium
Quote :
C. Daubeny found that the sulfate lost about a quarter of its contained
sulfur trioxide when heated for 3 hrs. at a bright red heat; and he stated
that if heated for a still longer time, the cold product gives a residue insoluble
in water, and which gives off some hydrogen sulfide when treated with acids.
J. B. J. D. Boussingault stated that "when small quantities are heated in the
blastflame, all the sulfur trioxide can be expelled."
H. Ie Chatelier stated that the decomposition of the sulfate begins at· 1160 °C.
A. S. Ginsberg gave 1120 °C for the melting point of molten magnesium sulfate, and
R. Nacken, 1124 °C. The latter stated that the salt is considerably decomposed
at its m.p.
E. H. Riesenfeld has studied the reduction, and found that there " are concurrent
reactions:" 2MgS04+C => 2Mg04 + 2S02 + C02, and MgS04 + C => MgO + S02 + CO.
C. Daubeny, Edin. Phil. Journ., 7. 111, ( 1822 )
J. B. J. D. Boussingault, Ann. Chim. Phys., ( 4 ),12. 419, ( 1867 )
H. le Chatelier, Bull. 8oc. Chim., ( 2 ), 47. 300, ( 1887 )
A. S. Ginsberg, Zeit. anorg. Ohem., 59. 346, ( 1908 ); 61. 122, ( 1909 )
R. Nacken, Gött. Nachr., 602, ( 1907 )
E. H. Riesenfeld, Journ. prakt. Chem., ( 2 ), 100. 115, ( 1920 )
______________________________________
The following is excerpted from Handbook of Inorganic Chemicals Pradyot Patnaik, Ph.D.
Physical Properties
The anhydrous salt consists of colorless rhombohedral crystals; density
2.66 g/cm3; decomposes at 1124°C; dissolves in Water , Ethanol , Glycerol;
sparingly soluble in Ether (1.16 g/mL at 18°C); insoluble in Acetone.
Epsom salt, or heptahydrate MgSO2•7H2O, constitutes colorless monoclinic
or rhombohedral crystals; refractive index 1.433; density 1.68 g/cm3; loses
six molecules of water of crystallization at 150 °C and becomes anhydrous
on prolonged heating at 200 °C.
Reactions
The anhydrous salt decomposes at elevated temperatures to Magnesium
Oxide, oxygen, sulfur dioxide, and sulfur trioxide. The decomposition commences
around 900 °C and is complete at about 1100 °C. The overall reaction is:
3 MgSO4 => 3MgO + O2 + 2 SO2 + SO3
(* My Note * This temperature is so much higher than what is needed for the
oxidation of SO2 , which is reverable , explains why dissociation occurs. For the
manufacture of Sulfuric acid SO2 is oxidised to SO3 by a catalyst at between
400 and 600 °C. )
Anhydrous MgSO4 may be reduced to Magnesium Oxide when heated with
carbon at 750 °C: MgSO4 + C => MgO + SO2 + CO
Double decomposition, in Sodium Hydroxide precipitates Magnesium Hydroxide
MgSO4 + 2NaOH => Mg(OH)2 + Na2SO4
____________________________________
Where do I send you my bill for research time ?
.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Oddly I don't quickly come up with a good reference for this. But thermal decomposition of MgSO4 requires temperatures of 900-1000 deg C to proceed at
a reasonable rate. Whether
MgSO4 -> MgO + SO3 or
2MgSO4 -> 2MgO + O2 + 2SO2
predominates, I don't know. If you are looking for ways to produce either SO2 or SO3 I would think there are easier ways.
|
|
|
Devilinajolie
Harmless

Posts: 17
Registered: 16-3-2009
Member Is Offline
Mood: No Mood
|
|
Thanks a lot franklyn your answer is very complete and thanks bbartlog
So :
At 900-1100°C you have :
3 MgSO4 => 3MgO + O2 + 2 SO2 + SO3
Is this reaction really mean you can produce sulfuric acid without any catalyst ?
[Edited on 6/13/2010 by Devilinajolie]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
FeSO4.7H2O (green vitriol) gives up its SO3 at a lower temperature than MgSO4 and was used to prepare H2SO4 before the "chamber process" was
developed.
Because of this sulphuric acid was once known as "oil of vitriol".
[Edited on 13-6-2010 by hissingnoise]
|
|
|
Devilinajolie
Harmless

Posts: 17
Registered: 16-3-2009
Member Is Offline
Mood: No Mood
|
|
Thanks a lot hissingnoise is there other reactions without catalyst like MgSO4 and FeSO4 who can produce sulfuric acid ?
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ Devilinajolie
You really must learn how to do your own research
Once again , the very first place to look _
http://en.wikipedia.org/wiki/Sulfur_trioxide
Look at Preparation
" Sulfur trioxide can be prepared in the laboratory by the two-stage pyrolysis
of sodium bisulfate. Sodium pyrosulfate is an intermediate product.
Dehydration at 315 °C:
2 NaHSO4 => Na2S2O7 + H2O
Cracking at 460 °C:
Na2S2O7 => Na2SO4 + SO3
This method will work for other metal bisulfates, the controlling factor being
the stability of the intermediate pyrosulfate salt."
* My Note *
Take specific notice of the temperature required for dissociation of
sodium pyrosulfate Na2S2O7 compared to MgSO4
The SO3 produced is suitable for fortifying commercial grade H2SO4
if that is your intent , which you have not as yet clearly stated.
http://www.sciencemadness.org/talk/viewthread.php?tid=13968
.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
There is no way that reaction will happen near 460 C, you need a lot more heat. Garage chemist in his thread on NaHSO4 to SO3 mentioned it was between
680-880°C.
I've heated NaHSO4 under the bunsen burner for several hours in a ceramic dish, and judging by the mass loss, it did not go beyond Na2S2O7. The
ceramic dish weakened and eventually broke during the prolonged heating. This reaction can not be done in glass without destroying it. Quartz or
equivalent must be used.
Btw, good to know about C reducing MgSO4 decomposition. Addition of C to CaSO4 also lowers decomposition temp. by 800 C (thus to 1200 C).
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
I regard Wikipedia as a starting point only. I invariably look for corroboration and
elaboration of what is stated there. CRC does not list Na2S2O7 , Wikipedia does
http://en.wikipedia.org/wiki/Sodium_pyrosulfate
Here is the download link to Garage Chemists own process description
perhaps he can comment for himself.
http://www.sciencemadness.org/member_publications/SO3_and_ol...
In defense of the higher temperature , excerpted below from
Treatise on General & Industrial Inorganic Chemistry
read in the last line.
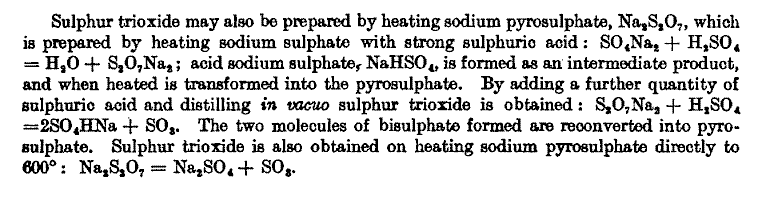
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Gmelin mentions decomposition begins at 460 C, but that at red glow the Na2S2O7 decomposes to Na2SO4 and SO3. Red glow is around the aforementioned
temperatures.
But if the decomposition of persulfate to pyrosulfate is as above, then all one would need is to add some H2SO4 to the pyrosulfate. Then the reaction
can be done at much lower heat and so in the suitable distillation apparatuses, as also shown here: http://www.youtube.com/watch?v=UrZ_sG07wGg It makes the raw, straight-forward decomposition trivial.
[Edited on 14-6-2010 by Formatik]
|
|
|
Devilinajolie
Harmless

Posts: 17
Registered: 16-3-2009
Member Is Offline
Mood: No Mood
|
|
Thanks a lot everyone , i'm so thankfull for your kindness .
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
OK, I got a phone call and for some seconds (less than 30 seconds), I over dehydrated my MgSO4.7H2O in a micro wave oven.
Based on subsequent reactions, I suspect I created MgSO4.xMgO, that is, a form of the basic magnesium sulfate salt as the latter contains Mg(OH)2 and
not MgO (arising from the thermal decomposition of MgSO4 as specified above).
Does anyone have a reference on such a salt existing and such a direct thermal path to its formation?
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
You assume this because it lost more mass then it should have, had it only dehydrated?
Apparently, 4.34 Mg(OH)2 · MgSO4 · x H2O exists [x = ~ 2] Per this:http://pubs.acs.org/doi/abs/10.1021/cm000249f But that's not really what you're looking for.
Are you sure it contains Mg(OH)2, which is (obviously) only possible if the sulfate decomposed before all the water - I can't imagine that
happening, as any SO3 would react very fast with Mg(OH)2.
MgSO4 · 7 H2O first decomposes to MgSO4 · H2O at around 150 degrees C, so perhaps: MgSO4 ·
H2O → Mg(OH)2 + SO3, but again, this reaction goes against everything I've learned, and seems very unlikely because
it would have to happen at less than 250°C (before the sulfate dehydrated completely).
Do you think the microwaves may have caused some otherwise impossible reaction?
[Edited on 5-8-2014 by Zyklon-A]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
The newly created salt in water produces a clearly insoluble white compound.
Could just be MgO from a partial thermal reduced MgSO4.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
I just got an idea, when I saw this topic!
So, could I just decompose CuSO4 by heat and then direct SO3 gas into water to make H2SO4? 
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
According to Wikipedia, yes, if you could heat it to 650°C. It would be a very impractical, expensive, and energy consuming process.
Also, I see that you're brand new here, but please, in the future, just look it up for yourself before asking on here. It took me about 30 seconds to
find the information from Wikipedia, which I'm sure you are also capable of doing.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Quote: Originally posted by zts16  | According to Wikipedia, yes, if you could heat it to 650°C. It would be a very impractical, expensive, and energy consuming process.
Also, I see that you're brand new here, but please, in the future, just look it up for yourself before asking on here. It took me about 30 seconds to
find the information from Wikipedia, which I'm sure you are also capable of doing. |
Are you sure? I think it's 560°C, not 650°C.  I've checked this before on
wikipedia, yes... ...but I needed to make sure, that I'm right I've checked this before on
wikipedia, yes... ...but I needed to make sure, that I'm right  And thanks And thanks 
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I just looked again, and while in the infobox it says 560°, the main body of the page says 650°. I took the time to look it up in the CRC handbook,
which has it as 650°, so the 560° must have been a typo.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Quote: Originally posted by zts16  | | I just looked again, and while in the infobox it says 560°, the main body of the page says 650°. I took the time to look it up in the CRC handbook,
which has it as 650°, so the 560° must have been a typo. |
Ok, maybe. 
|
|
|