| Pages:
1
2 |
thorazine
Harmless

Posts: 27
Registered: 4-3-2009
Member Is Offline
Mood: No Mood
|
|
How to convert ferrovanadium (82%) in vanadium pentoxide
Hi! I bought some ferrovanadium in ebay and what i need is vanadium pentoxide. Anyone know any route or literature where i can find a way to extract
and purify vanadium? Thanks!
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Mmmm...
Dissolve in sulfuric, filter impurities, base extract and oxidize, filter Fe(OH)3 and impurities, precipitate V2O5? Or crystallize sodium vanadate.
Tim
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Actually, use hydrochloric acid to get it into solution, it's better at dissolving the iron and forms soluble vanadium oxychlorides. You can use tech
grade or hardware store HCl as the main contaminate is iron, hardly a concern in this case; dilute so as to be 10% to 20% HCl.
Once you've done that filter the solution, then treat it so as to oxidise all the iron to Fe(III) and all the vanadium to V(V). You could slowly
bubble Cl2 through the warm solution, or bring it to near boiling and drip in fairly concentrated H2O2 (25% to 35%); the solution will change from a
greenish to a yellow to yellow-orange as this is done. Calculate the needed amount of Cl2 or H2O2 based on the assumption that all the iron is
originally Fe(II) and all vanadium as V(IV), use a distinct excess. Finish off by boiling the solution for a few minutes.
Then use Na2CO3 to neutralise the solution, the remaining HCl and the FeCl3 and the VOCl3, and add enough excess of Na2CO3 so as to dissolve the
vanadium as sodium metavanadate NaVO3. You can calculate the excess amount based on 18 g anhydrous Na2CO3 to 25 grams V2O5 or 14 grams of vanadium in
the ferrovanadium, plus however much would neutralise off the HCl you started with. There should be about 125 ml of water for every 18 g Na2CO3 (14 g
V) going to making NaVO3 so add more water if the filtered acid solution is more concentrated than this (in terms of grams V to ml H2O). Keep the
solution gently boiling will doing this, both the keep the NaVO3 in solution and to drive off CO2 - as the Na2CO3 is added the CO2 will create foaming
so add it slowly and stir after each addition. After all the Na2CO3 has been added, boil for several minutes more.
Filter the hot solution to remove the ppt of iron hydrated oxides. If the filtrate is not clear, run it through the same filter again as needed to
remove any solids.
At this point you can simply drop the pH to around 2 to get V2O5. However the V2O5 prepared this way is often somewhat colloidal and difficult to
retain on a filter, so the following alternative may be better.
Allow the solution to cool to 60-70 C. Now add a strong solution of an ammonium salt, 75 g of NH4Cl in 125 ml of hot water for every 14 g of
starting vanadium (multiply as needed to get the matching weight of ferrovanadium) Ammonium sulfate or nitrate can also be used, the amount of water
is adjusted so as to insure solution of the ammonium salt and the corresponding sodium salt - don't forget the amount of water in both the ammonium
and vanadium solutions. Mix well, then cover and set aside to cool for at least 6 hours. Collect the pale yellow crystals of NH4VO3 on a filter,
then wash with first a small amount of the ammonium salt solution, them with several small amounts of ice cold distilled/deionised water - until no
more chloride can be detected in the wash water if you've AgNO3 to test with. Allow the NH4VO3 crystals to air dry. Yield should be about 70% of the
vanadium in the ferrovanadium.
You can heat the ammonium metavandate to 60-670 C, with stirring, in air to get V2O5. The NH4VO3 is a useful reagent on its own, as are the sodium and
potassium metavanadates.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Or you could just buy some reasonable quality V2O5 from a pottery shop, it's used as a yellow pigment in glazes.
I've never tried dissolving ferrovanadium in strong HCl but it sounds like a slow process, even at 20 % HCl. Let us know how you get on.
What do you want the V2O5 for?
|
|
|
thorazine
Harmless

Posts: 27
Registered: 4-3-2009
Member Is Offline
Mood: No Mood
|
|
Thank you all!
not_important, i will try that this week! Thank you very much, i'm a beginner and your post is very detailed, its a big help!!  I just have H202 3%, and you said that you need at least 22%. Here I have sodium
hypochlorite (13%), will work as an oxidant? I found this paper* where they use it in oxidative precipitation. I just have H202 3%, and you said that you need at least 22%. Here I have sodium
hypochlorite (13%), will work as an oxidant? I found this paper* where they use it in oxidative precipitation.
* http://rapidshare.com/files/387794634/Recovery_of_vanadium_f...
I don´t have a fumehood, but i have this air extractor (300m2/h). I think if i put it over the bench there will be no problem, right?
An i don´t have NH4Cl but i have Ammonia (25%) and HCl, i think i can prepare it with this.. 
Thanks, again!
blogfast25,
in portugal is hard to find, and online i didn't find V2O5. I have no real purpose. I like chemistry as i like metals, found it a good subject to
start playing. I've tried to prepare FeCl3, but failed - i've just get a FDA approved Pigment Yellow 42 http://www.sciencemadness.org/talk/viewthread.php?tid=13794 ! Now i bought 250 g ferrovanadium in ebay. Maybe I'll convert 50g of Fev in ammonium
metavandate and V2O5. Then, i will thhink in what I can explore more, I am searching for info on V what i can do with it, to learn and have fun  . I have noticed that I can do with ammonium metavandate Mendelin Reagent. Do you have
more sugestions of things i can do with it? . I have noticed that I can do with ammonium metavandate Mendelin Reagent. Do you have
more sugestions of things i can do with it?
Thanks!
|
|
|
thorazine
Harmless

Posts: 27
Registered: 4-3-2009
Member Is Offline
Mood: No Mood
|
|
Humm..iwhat i said is wrong, in paper they said they used NaClO3 and not NaOCl!But i was searching and i have maybe 40g of Sodium chlorate ..can use
that?
[Edited on 16-5-2010 by thorazine]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The problem with using more dilute oxidants is that you drop the concentration of vanadium in the solution, this is particulary true with your H2O2.
Ammonium metavanadate is somewhat soluble, even in solutions saturated with ammonium salts. So too dilute means both leaving more vanadium behind in
solution, and having to use more ammonium salt to precipitate the portion of the vanadium that can be forced out of solution.
The paper is targeting V2O5 as the precipitated substance, and it is much less soluble than the vanadate. But they're talking about commercial
production where they can do a better job of filtering than you likely can.
You could go through the steps up past precipitating and filtering off the iron, using one of the oxidisers you have access too. Right off the top of
my head I don't know if hypochlorite will do the oxidation - you could try it on a small scale, if if pushes the vanadium to the +5 state you'll see
V2O5 dropping out of solution if the pH i in the 1-6 range. It might well work, as it will make Cl2 by reacting with the excess acid. Anyhow, once
the iron is gone you could evaporate down the solution until it's just damp (steambath or similar heat source) and then add water to make a solution
with the proper concentration of vanadium. The sodium metavanadate is stable, but doesn't crystallise readily and is somewhat hygroscopic so it's not
too convenient of a compound for storing. Making the NH4VO3 gives a stage of purification, and is handy for storage.
If your HCl is technical grade, not water-white in appearance, distill it into aqueous ammonia to make the NH4Cl, you don't want to be adding iron
back into the vanadium.
woelen has done a few things with vanadium, such as this http://81.207.88.128/science/chem/exps/vanadium/index.html
A note - while NaOH can be used to neutralise the acidic solution from dissolving the ferrovanadium, Na2CO3 has an advantage related to purification.
The likely contaminates in the ferrovanadium are C, Si, Al, and Ca. Carbon isn't going to dissolve, likely remaining as a mix of carbon and metal
carbides, but Si and Al can be brought into solution by strong base and Ca(OH)2 is slightly soluble. Using Na2CO3 pretty much avoids those problems.
As for the air extractor, I'll let someone else comment on that but it sounds rather small to me; do a forum search on "fumehood". If you avoid open
containers of HCl in concentrations above 20%, and don't use Cl2 or hypochlorite with acid, you should be OK. The fumes from more concentrated HCl
are hard on most metals, tools rust like crazy near it, and the fumes could damage the air extractor.
Remember that vanadium is somewhat toxic, although not persistent.
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I'm fairly sure that, under alkaline conditions, air will oxidise Fe to Fe(III) which will precipitate and V to V(V) which will dissolve.
Air is generally considered to be cheap and of low hazard.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The iron in particular will, but air oxidation can be slow and the reference procedures that I've seen never rely on it. Part of the problem with air
oxidation is that both iron and vanadium will have precipitated to some degree before conditions are alkaline enough to get decent oxidation rates,
and the resulting solid mixtures tend to be slow to fully oxidise and leach out. The other likely contaminates don't help either.
And I selected the more common oxidants. Several procedures for NH4VO3 or V2O5 from crude V2O5 or ferrovanadium use KMnO4 as the oxidiser, others use
HNO3. Both are harder to get now than back when the procedures were written, while the pool and spa industry has made it fairly easy to be able to
generate chlorine.
Because I've never read or do such a process, I can't say "do thus and thus and thus, and most of the vanadium will be in solution while all the iron
will have been removed." If I were to try it, I'd slowly drip the acid solution of V+Fe into a hot solution of Na2CO3 that was being strongly
aerated, and stirred as well to prevent buildup of a precipitate layer on the bottom. If they have both a mechanical stirrer and an air pump that
doesn't contaminate the air with oil, then it certainly could be tried.
|
|
|
thorazine
Harmless

Posts: 27
Registered: 4-3-2009
Member Is Offline
Mood: No Mood
|
|
not_important,
thanks a LOT! 
I was testing if a solution NaClO3 worked as an oxidant, but I got stuck on the first step. Added approximately 80 mL of HCl (% 20) at 8.11 g FeV,
about an hour on a magnetic stirrer and not dissolving. Perhaps the process is very slow, as blogfast25 said. I feel the presence of a funny smell, so
something must be happening .. 
[Edited on 17-5-2010 by thorazine]
|
|
|
woelen
Super Administrator
        
Posts: 8079
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Yes, dissolving of ferrovanadium is very very slow. Ferrovanadium is known for its corrosion resistance and its strength (for these reasons it is used
in better quality tools).
You could try adding some solution of NaOCl to the HCl (just a few drops, no gas should escape) and see if this helps. It also helps if you hit the
ferrovanadium into small particles with a heavy hammer. Add the NaOCl very slowly, dropwise, while stirring. Let us know if this helps. Another option
is strong heating (boiling) of the 15% solution of HCl. Beware of the nasty fumes from this boiling solution, do this outside. But in any case,
patience will be needed.
The method of non_important for working up the solution indeed should work. The only point is that if you use H2O2 that a brown peroxo complex is
formed at low pH, which is not that unstable and it may require quite some time of boiling before all of it is destroyed (this brown complex is much
more stable than the similar deep blue chromium complex). You must destroy this complex before adding a base, so keep on boiling until the dark
redbrown color of the complex is gone and the solution has turned more yellow than brown.
[Edited on 17-5-10 by woelen]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Thanks, woelen. And I'd forgotten how stable the perox complex can be.
Your ferrovanadium may have a higher percentage of vanadium than the stuff I worked with, and/or less aluminum, and/or more silicon. Pure vanadium
isn't appreciably attacked by HCl, but the alloy I had did dissolve in warm HCl.
The alternative dissolution methods use hot HNO3 + H2SO4, or hot HNO3 + HF; the second isn't something you want to mess with.
Try heating and adding small amounts of H2O2 or HNO3, or do as woelen suggested using NaOCl; they are all based on getting small amounts of oxidiser
into the solution (but not too much at once). In all cases there will be some chlorine given off, so have good ventilation, and if you do heat it to
boiling then there's the HCl fumes too. If you heat that much then it is best to do it in a Erlenmeyer flask to keep in spray and act as a condenser.
[Edited on 17-5-2010 by not_important]
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Incidentally, I know you can extract Fe(III) from solutions containing a lot of HCl using ether as an extraction solvent. Does vanadium extract?
I realise this is only useful if you can get ether but I's still like to know if the vanadium extracts too (take your pick of vanadium's oxidation
state, provided it's compatible with Fe(III) compounds)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | Incidentally, I know you can extract Fe(III) from solutions containing a lot of HCl using ether as an extraction solvent. Does vanadium extract?
I realise this is only useful if you can get ether but I's still like to know if the vanadium extracts too (take your pick of vanadium's oxidation
state, provided it's compatible with Fe(III) compounds) |
In such solutions, Fe(III) would be present as
[FeCl4]-. Although ether is polar, enabling ionic species to have some solubility in it, it is aprotic, meaning that the Fe(III) will not be
hydrolyzed by it.
But vanadium is both more electropositive than Fe and more stable in higher oxidation states. If it is present in solution in the (V) state (which is
most common in ordinary conditions), usually as the oxy-anion [VO4]---, strongly acid solutions are liable to hydrolyse it to polymeric anions and
then precipitate it out as V2O5. If it can be gotten into solution from ferrovanadium in a strong HCl solution, it is most likely to be in the (III)
oxidation state (Fe would initially be in the (II) state until further oxidized), which may be similarly as [VCl4]-; this would be less stable with
respect to oxidation than [FeCl4]- but less liable to hydrolysis.
The main industrial use of vanadium, other than in high-strength ferrous alloys such as used in tools, is as solid V2O5 catalyst in the manufacture of
H2SO4 via SO2 from SO2 gas. Do you want to make H2SO4 by this method? In this industrial reaction, the V2O5 is reduced to VO2 by the SO2 gas (from
combustion of sulfur, obtained from mining (Frasch process mostly) or by recovery in refining from S compounds such as thiophene in crude oil), which
is oxidized to SO3, and then the VO2 is oxidized back to V2O5 by heating in O2.
|
|
|
woelen
Super Administrator
        
Posts: 8079
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
JohnWW, it is not true that vanadium in strongly acidic solution would be in the +3 oxidation state. That oxidation state is very strongly reducing.
Especially if the help of some oxidizing agent is needed to get the vanadium in solution, it almost exclusively will be in the +5 oxidation state. In
20% HCl this will be in solution as a cationic species, the light yellow so-called pervanadyl ion, VO2(+), which must not be confused with the bright
blue vanadyl ion VO(2+). The name 'pervanadyl' is bad, because this has nothing to do with peroxide or peroxo complexes, the prefix 'per' just tells
that it is in a higher oxidation state than vanadyl (which has vanadium in oxidation state +4).
Vanadium in oxidation state +5 has a very complicated aqueous chemistry. At very high pH (around 14), it mainly exists as colorless VO4(3-), the
orthovanadate ion. When pH is lowered, this ion is hydrolyzed and species like VO2(OH)2(-) are formed, which when crystallized from solution forms the
metavanadate ion VO3(-), but the metavanadate ion probably is not a single simple ion, but more like a polymeric species [VO3)n](n-), very similar to
the metaphosphates (calgon). When pH is reduced further, then further hydrolysis and condensation occurs, anionic species are formed with many
vanadium atoms in it, with bridging O and OH groups. At pH in the range 2 ... 3 (IIRC) the hydrolysis and condensation becomes so strong that
macroscopic neutral particles are formed, which can be formulated like nV2O5.mH2O, with n and m being very large. This is a deep orange/red
precipitate. When pH is lowered further, then further protonization and subsequent splitting off of water occurs which leads to formation of deep
orange polyatomic cationic species but finally at pH below 0 (strong acid at fairly high concentration) the splitting up is complete and simple
aqueous VO2(+) ions go in solution.
Some info with pictures: http://woelen.homescience.net/science/chem/solutions/v.html
[Edited on 18-5-10 by woelen]
|
|
|
thorazine
Harmless

Posts: 27
Registered: 4-3-2009
Member Is Offline
Mood: No Mood
|
|
Yesterday when I added a few drops of sodium hypochlorite (13%) the solution turned bright green. I left about an hour and a half on the magnetic
stirrer, and not dissolved but the solution turned a green darker. Today when I see the solution was blue (as in the pic).

Uploaded with ImageShack.us
Right now I'm outside boiling the solution. When it reached the boiling point of the solution turned dark green again.
I have a question: as the solution is evaporating, as it boils, can i add water to keep the solution?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Yes, you can add water to maintain the volume. However the vanadium compounds formed have high solubilities, so some concentration will not cause
problems.
Normally the green colour means that the solution is mostly V(III), while blue is V(IV). Ferrovanadium will function as a reducing agent, when the
NaOCl had been consumed the vanadium in solution was being reduced, air can oxidise it. However aqueous VOCl2 in strong HCl is a bright green hue
even though it is a V(IV) compound. Heat can also change the coordination sphere, changing the colour.
|
|
|
thorazine
Harmless

Posts: 27
Registered: 4-3-2009
Member Is Offline
Mood: No Mood
|
|
Yesterday, after the solution being boiling about an hour i've get a red / brown solution and for what i read in woelen's site maybe its V4O92-.
Filter and today added sodium hypochlorite until the entire solution becomes a dark green. Today i'll boil the solution to concentrate (the actual
volume is maybe 350mL with 8,11g of FeV) and add Na2CO3 to neutralize the HCl and precipite Fe.
[Edited on 19-5-2010 by thorazine]
[Edited on 19-5-2010 by thorazine]
|
|
|
woelen
Super Administrator
        
Posts: 8079
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The brown ion V4O9(2-) only exists at high pH, in alkaline solution when vanadium is in the +4 oxidation state. So, if you have a red/brown color in
acidic solution, then you certainly don't have V4O9(2-). At low pH, vanadium in oxidation state +4 forms the bright blue (very much like copper ion as
in copper sulfate) vanadyl ion VO(2+).
|
|
|
thorazine
Harmless

Posts: 27
Registered: 4-3-2009
Member Is Offline
Mood: No Mood
|
|
Correct. I read more carefully and really could not be V4O9 (2 -). What is the justification for that color, then? It was exactly that red / brow like
in picture. However the solution is now dark green. I am boiling it to remove excess solvent because I have a small problem: the larger borosilicate
glass I have is an 250 mL Erlenmeyer, i need to work with small volumes. When I was adding sodium hypochlorite it formed an intense yellow color,
which after dissolving turned the green. The idea I have is to decrease the volume boiling and then add NaOCl (13%) to oxidize any Fe (II) to Fe (III)
and all V (IV) to V (V) - until the solution turn yellow, then.
|
|
|
thorazine
Harmless

Posts: 27
Registered: 4-3-2009
Member Is Offline
Mood: No Mood
|
|
I was experimenting and i add a solution of 10g of NaOCl3 in 15ml of water while boiling and it turned red/brown again, but a more bright red. And
the foam on the topo turned yellow..
[Edited on 19-5-2010 by thorazine]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
red-brown is often polymers of hydrated V(5) oxide, or mixed V(5)-V(4) Yellow is V(5) in solution at various pH values. Green can be solutions of
V(4)+V(5), of V(4) in high Cl(-) concentrations.
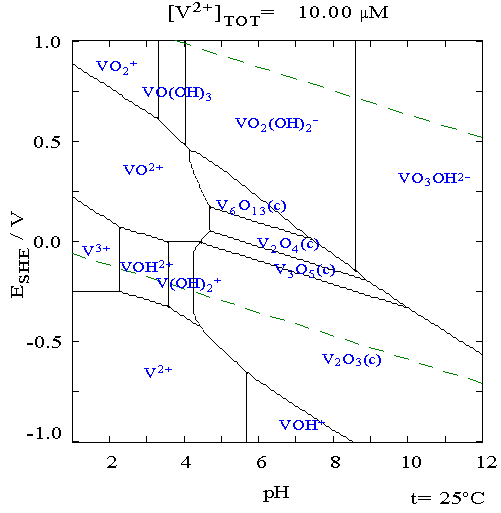
The upper dashed line marks where the stuff will oxidise water to O2, the lower dashed line where water is reduced to H2. Those reactions may be slow
for various reasons, and concentrations as well as the formation of complexes can shift things, but this should give you a feel for what species may
be present. You should be able to find a similar Pourbaix diagram for iron - such as is here: http://en.wikipedia.org/wiki/Pourbaix_diagram
|
|
|
thorazine
Harmless

Posts: 27
Registered: 4-3-2009
Member Is Offline
Mood: No Mood
|
|
When added NaOCl3 got a brown precipitate. Maybe V2O5 quite contaminated. I'll start again and do exactly as no_important said. Tomorrow I will try to
go to a drugstore to get H2O2 (30%).
Thank you a lot! I'm a newbie, thanks for your patient. I'm learning a lot .. 
|
|
|
woelen
Super Administrator
        
Posts: 8079
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
NaOCl3? What do you mean with that? You mean NaOCl? or Na2CO3?
V2O5, when precipitated from aqueous solution, has a beautiful bright orange color, as you might have noticed from the pictures in my webpage.
However, if iron in oxidation state +3 is present as well, I can imagine that a more dirty brown color is obtained and that the precipitate is a mixed
hydrated ferric/vanadium(V) oxide, which also could be formulated as some form of hydrated ferric vanadate.
I think that the only way to get rid of the iron is to add a large excess of NaOH, such that all vanadium goes into solution as orthovanadate, while
the iron precipitates as ferric hydroxide. The clear liquid then is decanted from the ferric hydroxide and then carefully acid is added, until the
orange precipitate of hydrous V2O5 is formed. In this way you might be able to recover most of the vanadium. Don't add too much acid, as that leads to
redissolving of the hydrated V2O5 and formation of VO2(+).
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I think it's better to use Na2CO3 than NaOH. Hydroxide could pull some of the contaminates into solution, depending what is the the alloy besides V
and Fe. The carbonate is commonly used in the various procedures I've read for getting pure vanadium compounds from the old style crude V2O5, and
for making NH4VO3. Aqueous ammonia is alkaline enough to do the job, but as that compound is not very soluble using ammonia directly doesn't do well
for purifying.
I think that NaClO3 was meant, it's been mentioned before.
|
|
|
| Pages:
1
2 |