| Pages:
1
2
3
..
6 |
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
help make white fuming nitric acid.
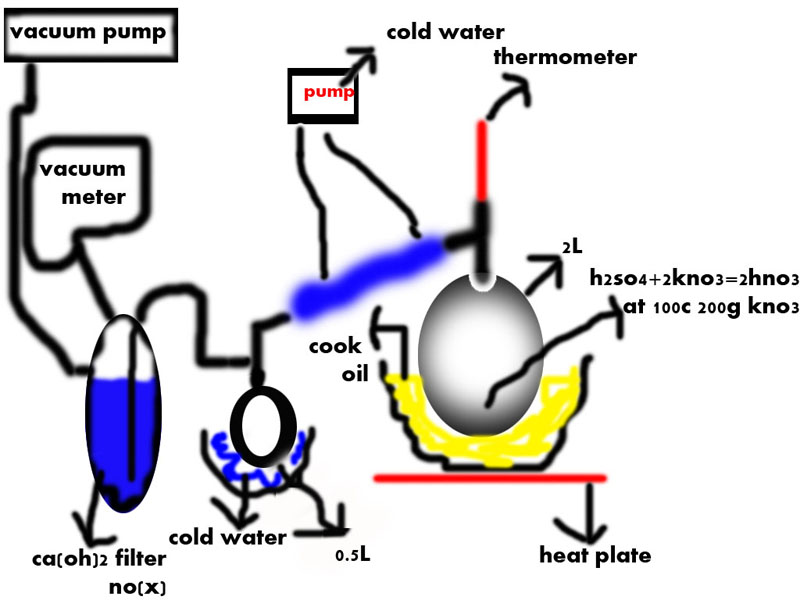
hello, i build a vacuum distillation system as shown on the picture below, i run this setup with oil temperature 140-120c, 210 gram kno3 and 106 gram
h2so4, i run it for 1.5 hour, the thermometer didn't change its was 28c, my lie-big condenser was 30cm long and already get water from ice-water bate,
i got alot of nitrogen oxides, and my yield was poor(about few drops), the nitrogen oxides bubble alot, at my ca(oh)2 filter, and i see alot of redish
vapor in 2L boiling flask and circulating white fumes inside it, i think because of my ca(oh)2 filter my system build little bit preasure 1-1.2 bar,
and all my hno3 fumes heavy so soo they circulate in 2L flask until they became oxides+water, is my theory true ?
i also try remove my bubbler , to allow the nitrogen oxides go rapidly to atmosphere and let my hno3 fumes climb to the condenser, i think its help
little, is it possible that the big size and spherical shape of my 2L flask cause the circulation+decomposition of my pure hno3?
i see in my setup that the bubbler don't work as good as i thought, i was able to see above my calcium hydroxide solution red fumes, which are
dangerous for my water aspirator, anyone have an idea how could i make trap for the nitrogen oxides, i cant get solid carbon dioxide.
and anyone know how i can help my hno3 fumes climb fast to my condenser?
Thanks for all who pay attention ! 
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Use equal weights of nitrate and H2SO4 and forget about vacuum distillation.
Your condenser and receiver aren't cold cold enough to condense the HNO3 vapours.
Receiver temperatures close that of dry-ice are needed to condense HNO3 vapours at aspirator pressures.
Without vacuum your HNO3 will be coloured by NO2 but this can be removed to a large extent by blowing dry air through it.
If you need water-white HNO3 just add a small pinch of urea as a last resort.
The bubbling in your filter, BTW, was caused by HNO3 vapours reaching the lime and being neutralised.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Amen.
I have distilled quite a bit of HNO3 over the course of years and found certain truths. You really don't need the vacuum.
The "sweet spot" of temp is 80 C - and a distinctly cold reception vessel. There is where you'll get some serious flow.
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
as i write above, i didn't use my vacuum pump in the experiment.
red fuming nitric acid is suitable for nitroglycerin synthesis?
so, if i use same weight of kno3 and h2so4 il spend valuable sulfuric acid, i read that i can use as twice kno3 by more heat:
http://www.lateralscience.co.uk/1888chem/experiments.html (look at the middle of the page)
so you say that without dry ice nitric acid vacuum distillation isn't practical look at this video, he didn't talk about dry ice:
http://www.youtube.com/watch?v=CtdX5YmOdcs
and this one make the acid without long condenser that i have:
http://www.youtube.com/NurdRage#p/u/48/2yE7v4wkuZU
is he use salt-water solution ? how he got batter results? salt-water solution can really help the condensation?
i see another man which use water-ice bath and successfully make hno3 with vacuum distillation:
http://www.craigsarea.com/hno3.html
how you can explain that he condense the product with only salt ice condenser under vacuum?
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
See Nitric Acid in Brauer - Handbook of Preparative Inorganic Chemistry
p. 491. La book is in the library.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
hissingnoise & quicksilver succintly stated the process shown in Brauer
Not having done this myself I only relate here what is written in older literature.
HNO3 is prepared by distillation of Sodium Nitrate with concentrated Sulfuric acid
in a cast iron retort forming Sodium Bisulfate and condensing the Nitric acid vapor
in a cold receiver. This is not easily done well without understanding the process
procedes in two main stages and the transitions in distillation along the way.
Distillation readily occurs just over 86 ºC , raising the temperature causes distilled
vapors to decompose thus , 4 HNO3 => 4 NO2 + 2 H2O + O2 , producing Oxygen
and Nitrogen dioxide which dissolves in the initial Nitric acid becoming yellow tinged
fuming Nitric acid.
Above 200 ºC Sodium Bisulfate further reacts with more Sodium Nitrate , becoming
Sulfate , liberating all the Nitric acid only as the temperature rises to 250 ºC.
If Sulfuric acid is used in excess , the highest concentration of Nitric acid distills
at lower temperatures becoming more diluted by water and even Sulfuric acid as
the temperature rises. Nitric acid is initially more concentrated because the water
is fixed by Sodium Bisulfate forming a hydrate which decomposes at 150 ºC.
If limited to the lower temperature , less Sulfuric acid is used than needed for
complete formation to Bisulfate to ensure the retention of water. To obtain fuming
Nitric acid , 2 mols of nitrate to 1 mol of Sulfuric acid is used for Sulfate to form as
the temperature elevates. See page 383, 385, 400, 401
A Treatise on General and Industrial Inorganic Chemistry
http://www.sciencemadness.org/talk/viewthread.php?tid=6664&a...
( Avoiding decomposition with use of HNO3 also has peculiarities that must be observed )
From footnote 1 on page 400 in the above reference _
" Whereas nitrous gases are readily expelled from nitric acid, they are not easily eleiminated
from the concentrated nitric-sulphuric acid mixtures commonly used in explosive factories,
and it is best to prevent their formation during the mixing of the striong nitric acid with the
sulphuric acid by cooling the mixing vessel outside with a spray of water and taking the pre-
caution to add the sulphuric acid to the nitric acid (not vice versa) and stir the mass well."
* My note * This is particularly troubling if nitration is done directly in the H2SO4 - nitrate salt
mixture, requiring extensive cooling , even though brown fumes inevitably result.
( The order in which ingredients are mixed can effect considerably the product yielded.)
Excerpted from page 2-14 of Military Explosives TM 9-1300-214
" During an inspection of a small Canadian TNT plant at Beloeil near Montreal in 1941,
LTC John P. Harris of Ordnance discovered that the plant was "doing things backward"
by putting toluene into the acid instead of putting acid into the toluene. Despite some
resistance by US TNT producers, the new process was tried at the partly built Keystone
Plant at Meadville, PA. The result was a trippling of TNT output. Lines designed to turn
out 16 tons a day produced more than 50 tons a day. The need for TNT substitutes
vanished, and the cost per unit was cut in half."
.
[Edited on 27-4-2010 by franklyn]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
There am being a large difference in usefulness — between stating and referencing.
Chemical engineering is in the details. A drawing or two helps.
Prudent
Proper
Planning
Prevents
Piss-poor
Performance
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by franklyn  | hissingnoise & quicksilver succintly stated the process shown in Brauer
Not having done this myself I only relate here what is written in older literature.
. |
I recommend :—
Allin Cottrell
Nitric Acid and Nitrates
Gurney and Jackson - Edinburgh 1923
454 pages.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Full title of citation here _ (" There am being a large difference in usefulness — between stating and referencing.")
http://books.google.com/books?id=Oz5DAAAAIAAJ
Not available in public domain , search for repository near you here _
http://www.worldcat.org/title/manufacture-of-nitric-acid-and...
.
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I have never had problems condensing nitric acid in a vacuum distillation, using simple tapwater in the condenser. You just have to include some way
to regulate the pressure in the system. I add a T-section between the pump and the rest of the system. A short length of rubber hose fitted with a
screw clamp is attached to the free branch of the T-section, and this is then used to regulate the pressure.
The screw clamp is then adjusted until nitric acid comes over at 50-60 C.
When the distillation is done, the screw clamp is slowly opened before the pump is turned off. This makes it easier to shut the
operation down without suckbacks or other nasty surprises.
Edit: I should just clarify that I never distil nitric from sulfuric and a nitrate, but rather from sulfuric and dilute nitric. It is easier for me to
buy dilute nitric acid than sodium- potassium- or ammonium nitrates.
[Edited on 27-4-2010 by Microtek]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Variations in water pressure would make an aspirator less easy to control than a vacuum pump.
But even at 60*C I'd expect some dissolved NO2. . .
Brauer uses 36-38*C!
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
i can control the water pressure my aspirator get with a valve, if il make 100g h2so4 + 100g kno3 in 0.1-0.5 bar pressure, using a water-ice bath for
condenser i will get my fuming white nitric acid? i ask it here ... because its hard for me to run my system, i don't have time to spend.
Thanks for all who help.
[Edited on 27-4-2010 by avi66]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
You can't control the variations in water pressure which sometimes occur in municipal water-supplies. . .
But I have used RFNA in MA to prepare nitro and alone to prepare RDX without incident.
WFNA is specified for PETN and this may be due to the increased danger of runaway using RFNA.
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
so, i read from alot of sources that vacuum distillation of nitric acid is practical with normal ice bath liquid in condenser, so if i use low
temperature(50c) with 0.1 atm preasure i will get wfna(kno3 + h2so4 = khso4 + hno3) ?
here is one:
http://www.craigsarea.com/hno3.html
franklyn, its a fact that nitric acid decomposes above 86c, so how this reaction khso4 + kno3=hno3 +k2so4) can occur at 200-250c without cause the thermal decomposition of the nitric acid? khso4 + kno3=hno3 +k2so4) can occur at 200-250c without cause the thermal decomposition of the nitric acid?
i think il try this experiment by myself after i recrystallized my khso4 which right now dehydrate under the sun force, i will put kno3 in molten
khso4, and check for nitric acid to came out.
in my lest experiment which i mentioned above, i used potassium nitrate which where slighty mixed with iron fertilizer, which make it slight pink
powder(really slight), it is possible that the impurities cause the formation of hno3 to decomposed to nitrogen oxides ?
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by avi66  | franklyn, its a fact that nitric acid decomposes above 86c, so how this reaction khso4 + kno3=hno3 +k2so4) can occur at 200-250c without cause the thermal decomposition of the nitric acid? khso4 + kno3=hno3 +k2so4) can occur at 200-250c without cause the thermal decomposition of the nitric acid? |
I'm not an authority I only in part quoted and referenced the source.
Concentrated acid fumes at room temperature indicating decomposition
is occuring , white if pure , red if there is nitrous acid contained. The
gas distilled is not acid per say but the equivalent of the fumes produced
when condensed and reconstituted. In actuality HNO3 is an aqueous
solution of the acid anhydride NOx ( nitrogen oxides ).
.
|
|
|
DetaDude
Harmless

Posts: 38
Registered: 21-3-2009
Location: Upper left hand U.S., Portland area
Member Is Offline
Mood: Alert
|
|
avi66
There are several threads on this forum dealing with this subject, by many very sharp minded chemistry people.
You may want to check out a few of these threads.
Try Our beloved nitric acid in General Chemistry (about pg. 5) this is just one on the subject there are many more excellent threads that deal with
this vital acid.
Genius creates many a great works............Labor alone finishes them!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
yada yada
|
|
|
Contrabasso
Hazard to Others
  
Posts: 277
Registered: 2-4-2008
Member Is Offline
Mood: No Mood
|
|
First this is a production process for a hazardous chemical it deserves your time, attention and respect. If you can't give that don't start - you are
too likely to get hurt.
To distil from dil nitric and conc sulphuric then distil under reduced pressure with a vapour temp about 60C and adjust the vacuum to hold this
steadily, Be certain that the vapours condense in the middle third of the condenser -the circulating water may need cooling, Keep the product receiver
cold -even iced to keep the product from boiling off!
|
|
|
medx
Harmless

Posts: 10
Registered: 20-4-2010
Member Is Offline
Mood: No Mood
|
|
I used a simple distillation system to get WFNA from reaction between H2SO4 and KNO3 or NH4NO3… I made this many times.
I reached some results and I have few questions.
First results;
1- I got concentrated nitric acids. I think, at the best results, it’s concentration is about 92-5%. In some experiments product concentration was
less than 90%.
2- In reaction for H2SO4/KNO3 mole ratio I used both 1mol/1mol and 1mol/2mol. If you need concentration bigger than 90% as yield of product there
isn’t any utility. According theory if you use ratio of 1mol/2mol you get 2mol HNO3. But in truth after you get 1 mole nitric acid more acid
decreased your acid concentration too much. For testing, after about 1 mole HNO3 occur I gathered remained acid in different container. Concentration
last acid was very low. Because for second mole you must increase temperature so HNO3 decompose.
3- I reached the best concentration results when I finished reaction when I reached about 80% of theorical yield. (This means 1mol/1mol give 1mol
HNO3).
4- I am not very sure but I think at first stage temperature of heater not so important. I say this for high temperature. Dropping start when
temperature of reaction solution is about 85C. if temperature of heater higher it drops very fast.
These are some result which I reached. If anyone ask detail I can answer how much I know.
My questions;
Quote: Originally posted by hissingnoise  |
Without vacuum your HNO3 will be coloured by NO2 but this can be removed to a large extent by blowing dry air through it.
If you need water-white HNO3 just add a small pinch of urea as a last resort.
|
1- Does Urea change only acid color? Or does it increase acid’s concentration at same time? I added it to acid, its color changed but I think it
decomposed acid. Because after this for example, acid didn’t react with hexamine.
2- What is the a pratical method for dry air?
3- What should I do to increase yield of acid which is concentration higher than 93%?
4- Are there anyone who try easier method to make WFNA?
I’m sorry I didn’t understand mean MA…
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Urea does not only change color, it removes NO2 from the acid, but also makes it somewhat more dilute. Urea reacts with NO2 to form water, N2 and CO2.
The gases escape from the acid, the water remains in the acid. If you have yellow acid it only contains a small amount of NO2 and then you only need a
small amount of urea and the urea only makes it slightly more dilute.
If excess urea is used, then urea nitrate is formed, which is sparingly soluble. You have to add just enough urea to make the acid colorless, do not
more than that amount.
Drying with air can be done with a little pump (e.g. aquarium pump) with a glass tube attached to it, which is immersed in the acid. Do not immerse
plastic or rubber tubes in the acid! I personally don't like this. If you don't do this carefully you may introduce dust and other impurities in the
acid and I also expect that you will have a lot of very nasty fumes of the acid going out of the bottle when air is bubbled through it. Of course you
could lead the outgoing gases through another tube and immerse that in a dilute solution of NaOH to absorb the nasty fumes. The tube, however, will be
eaten away quickly if this is plain plastic or rubber.
[Edited on 28-5-10 by woelen]
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
I prefer the „purging” with dry air. The pictures below are from my homemade apparatus, but the possibilities for improvisations are endless.
http://img192.imageshack.us/i/41043.jpg/
http://img188.imageshack.us/i/41053.jpg/
http://img135.imageshack.us/i/41074.jpg/
http://img412.imageshack.us/i/41088.jpg/
For maximum results you have to add a drying chamber for the air (in this case, plastic container with calcium chloride) and hot water bath for the
acid. The best part in this method (4NO2 + O2 + 2H2O => 4HNO3) is obvious, so I never tried to „clean” the acid with urea.
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
Jimbo Jones, how hot is the water bath you use in acid purging?
When logic and proportion have fallen sloppy dead...
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Around 50 – 60 °C. The starting nitric acid in the pictures (50 ml.) was absolutely clean after 40 – 45 minutes and the volume was reduced only
to 46 – 47 milliliters. The same acid was used directly in the production of RDX. The yield was around 12 gr. from 20 gr. HDN.
|
|
|
medx
Harmless

Posts: 10
Registered: 20-4-2010
Member Is Offline
Mood: No Mood
|
|
Jibo Jones, did you measure its concentration? How did it change?
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
I don’t have precise scales (or desire for titration), but I’ll bet it’s over 90 % for sure. The RDX yield was given as comparison for the
people who are familiar with the process.
|
|
|
| Pages:
1
2
3
..
6 |